medK launches needle-free extension lines for vascular access

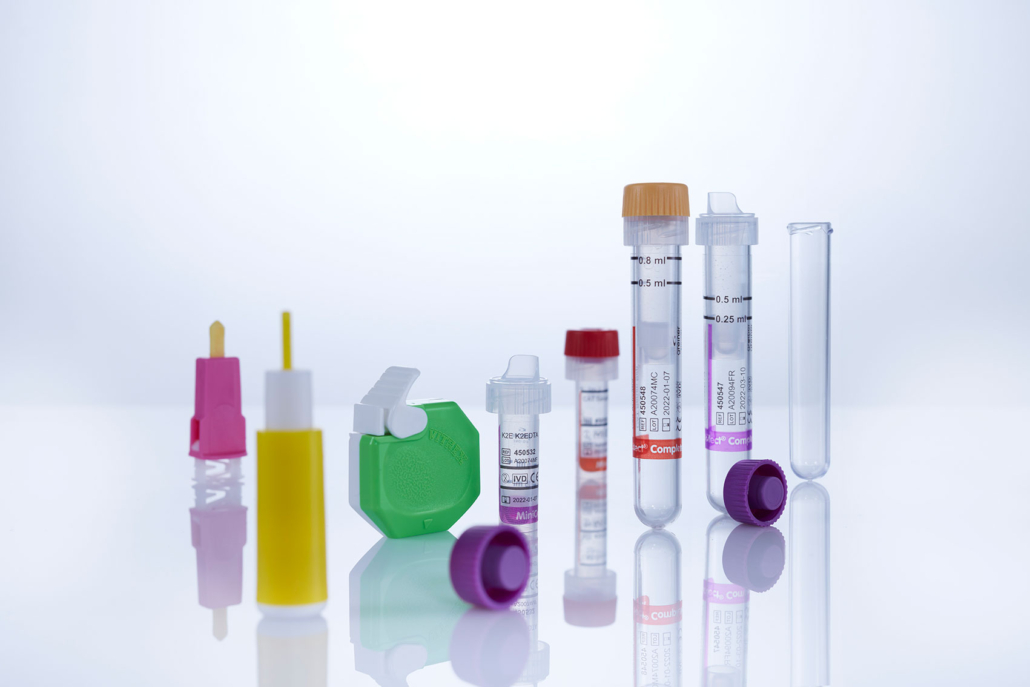
The needle-free extension line with triple valve. photo: ©medK GmbH
medK, a German company specialising in interventional products, has launched a needle-free solution for vascular access. The needle-free connection offers secure and fast access to fluid lines eliminating the risk of needle sticks. The extension line can also be connected with a glass syringe. With the multi-way versions there is no need to use a stopcock or a manifold.
medK Needle free Extension Lines are compatible with disinfection with IPA wipes (Isopropanol). This and the smooth valve surface allows easy disinfection and reduces the risk of infections. The leak free disconnection protects against blood contaminations for health workers. Once disconnected it provides reliable closure and can stay up to seven days or two hundred activations with the patient. The fully visible flow path allows visual checking of the fluid.
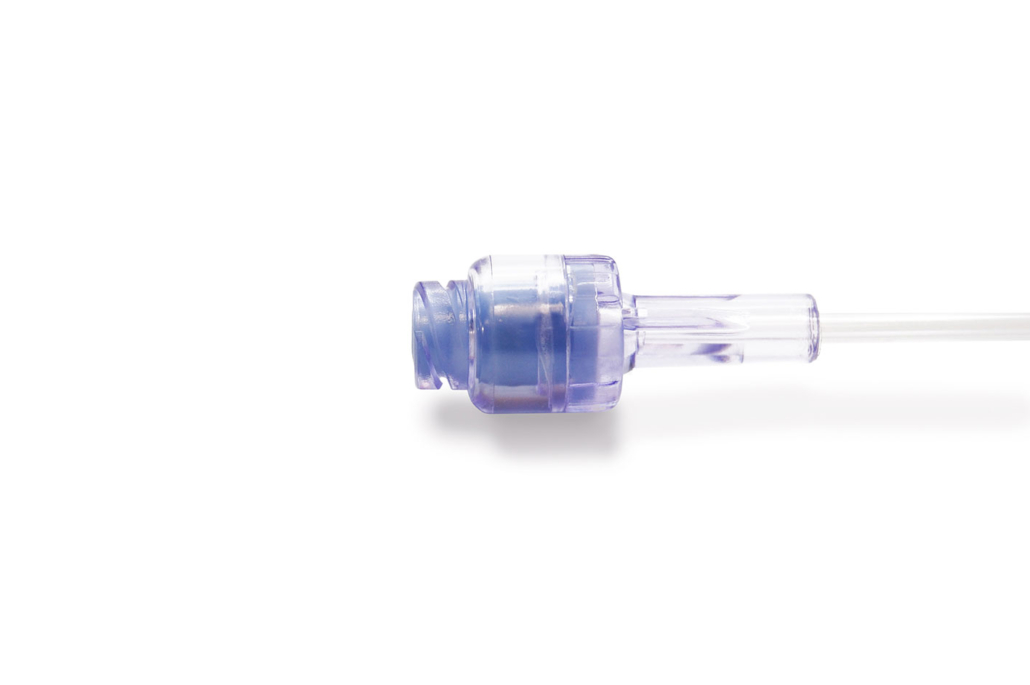
The needle-free extension line valve. Photo: ©medK GmbH
The needle-free extension lines are also available with a combined check valve that safeguards against back tracking. The needle-free extension lines are compatible with Isopropanol, Chlorohexidine, blood and lipids. The device is suitable for use in MRI procedures and is latex and animal product free.
medK extension lines are available as single, dual and triple access with needle free or combined check valve.
- For further details, visit: med-k.com