Chest CT software
AI-Rad Companion Chest CT, an intelligent software assistant for radiology, was recently
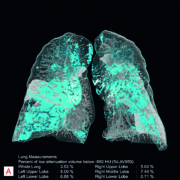
awarded the CE mark. This artificial intelligence (AI)-based software helps radiologists interpret CT (Computed Tomography) images of the thorax faster and more precisely, and to document the findings in less time with the help of automatic measurements. AI-Rad Companion Chest CT is the first AI-based application on the new AI-Rad Companion platform, and is vendor-neutral, which means the software can evaluate image data from many CT system manufacturers. Siemens Healthineers plans to expand this platform so that more and more intelligent algorithms will be available for additional organs and modalities. This will enable the company to consistently expand its portfolio of effective solutions for AI-based clinical decision support. Results drawn up by radiologists can vary by some 10-20 percent, depending on the investigator. Algorithm-based diagnostics completely obviate this variability, generating constant results. That alone is a huge advantage of AI platforms like AI-Rad Companion. Using CT images of the thorax (chest), the software can differentiate between the various structures of the chest, highlight them individually, and mark and measure potential abnormalities automatically. This applies equally to organs such as the heart and lungs, aorta, and vertebral bodies. The software automatically turns the findings into a quantitative report, which can be called up via the image viewing system used by the radiologist in the clinical routine. In certain circumstances, the intelligent assistant also alerts physicians to potential abnormalities that would otherwise have been missed because they were not the focus of the original examination, e.g. chance discoveries of pathological dilations of the aorta (aneurysms). Examination using a chest CT is a procedure often used in everyday clinical practice. For radiologists, this means more examinations in a limited amount of time and usually for low reimbursement rates. AI-Rad Companion Chest CT is a tool that can actually simultaneously increase productivity and quality in diagnostic radiology. AI-Rad Companion Chest CT is a cloud-based solution and uses certified, secure teamplay infrastructure that complies with the Health Information Portability and Accountability Act (HIPAA) in the U.S., and with the General Data Protection Regulation (GDPR) in the EU. The software conforms to Digital Imaging and Communications in Medicine (DICOM) standards. The images and all supporting information can be made automatically available in the picture archiving and communication system (PACS) in line with the radiologist’s individual requirements.
Read more