Continuous critical care patient monitoring during in-hospital transport
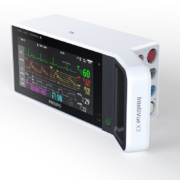
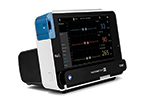
Philips IntelliVue X3, a portable monitor with an intuitive smartphone- style operation, enables continuous monitoring during transport to support a more complete data record.
When patients are transferred from one department to another, clinicians often struggle with incomplete data records leading to inefficiency due to multiple systems operating independently. Getting a complete view into patient data to allow for informed clinical decision support requires manual steps and can contribute to a higher potential for error, especially when dealing with critical care patients. IntelliVue X3 integrates seamlessly into the existing IntelliVue Patient Monitoring system, including bedside and transport monitors, the clinical network and the Philips family of central stations. Data from the IntelliVue X3 is also integrated into mobile applications, the hospital network, and interfaces that connect the system to other medical devices and to the hospital’s electronic medical record (EMR) system. As part of this larger patient monitoring portfolio, X3 assists clinicians in providing the best possible care for patients across all levels of acuity and supporting institution-wide standardization. Health systems need accurate and transparent information and processes to help clinical staff make faster, more consistent decisions based on patient conditions and history. With the development of the IntelliVue X3, the aim is to ensure that data isn’t being lost during transitions so that clinicians are provided with all the information they need, when they need it. In addition to delivering complete data records, IntelliVue X3 provides a comprehensive and scalable set of clinical measurements, ranging from basic to advanced monitoring. It allows for advanced monitoring during stationary and transport situations, including dual invasive blood pressure, built-in mainstream CO2, a choice between three SpO2 technologies and state-of-the-art ECG/arrhythmia. The IntelliVue X3 has received the CE Mark in Europe. Pending 510(k), it is not available for sale in the U.S.
Read more