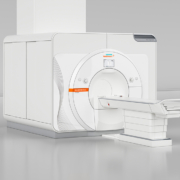
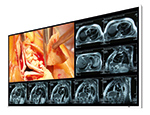
Siemens Healthineers has achieved CE approval for the 7 Tesla magnetic resonance (MR) scanner Magnetom Terra, making it the first-ever ultra-high-field MR scanner to be approved for clinical use. This development is now establishing 7T imaging in the clinical routine and expanding the scope of diagnostic MRI – 15 years after 3T scanners first became established. With this new clinical field strength, it is possible to achieve a new level of detail in anatomy and function, helping further pave the way for precision medicine. Thanks to its very high spatial and spectral resolution, Magnetom Terra provides detailed insights into the human musculoskeletal system, presents a precise picture of the metabolic processes in the brain and also aids in the visualization of neurological diseases such as Alzheimer’s, epilepsy, and multiple sclerosis (MS). The advantages of ultra-high-field imaging are especially apparent in brain imaging. At 7T, lesions can be identified more clearly thanks to the higher resolution and stronger image contrast. One example of this is the examination of epilepsy patients, where the clearer distinction between white and grey matter opens up new diagnostic capabilities, which are not possible at lower field strengths. Results from 7 Tesla can also be beneficial to patients with MS by improving the visibility of lesions in the grey brain matter that can lead to cognitive impairments. Here, the combination of a better signal-to-noise ratio, stronger tissue contrast and greater spatial resolution means that 7T can reveal information that would be invisible at 3T. With Magnetom Terra’s Dual Mode functionality, users can easily switch between the clinical protocols and innovative research methods. This makes it an optimal platform for translational research, allowing the use of 7T to be expanded, such as for whole-body applications. Until now, 7T has typically been used to examine and enhance the visibility of extremely small pathologies with anatomical imaging, as well as sub-cortical brain activations utilizing functional imaging. In the near future, the exploration of metabolic changes in the patient will play an important role, and 7 Tesla could be thought of as a MRI microscope that examines the anatomy, function, and metabolism of body tissue. Furthermore, the open system architecture of Magnetom Terra is especially attractive for researchers, allowing them to utilize and build on their own developments. Manufactured at Siemens Magnet Technology in Oxford, England, the actively shielded magnet on the new ultrahigh- field MR system is the lightest 7 Tesla whole-body magnet in the world, being 50 percent lighter than previous actively shielded magnets. The low total weight of Magnetom Terra facilitates its installation in the clinical environment. It uses the same software platform as other clinical scanners from Siemens Healthineers in the 1.5T and 3T segments and is closely modeled on their established user interface. In conjunction with specially optimized applications for the 7 Tesla system, this allows easy operation of the ultra-high-field system in clinical routine, as well as the easy exchange of study protocols across other MR systems. Magnetom Terra’s current FDA approval status is 510(k) pending.
Read more