Micro-device could pick up early signs of heart attack or stroke
Heart attacks and strokes are the world’s leading cause of death
Heart attacks and strokes are the world’s leading cause of death
A new joint guideline published 1 April from the American College of Cardiology, the American Heart Association, and the Heart Failure Society of America, increases the focus on preventing heart failure (HF) in people who are showing early signs of “pre-heart failure,” and updates treatment strategies for people with symptomatic heart failure to include SGLT-2 […]
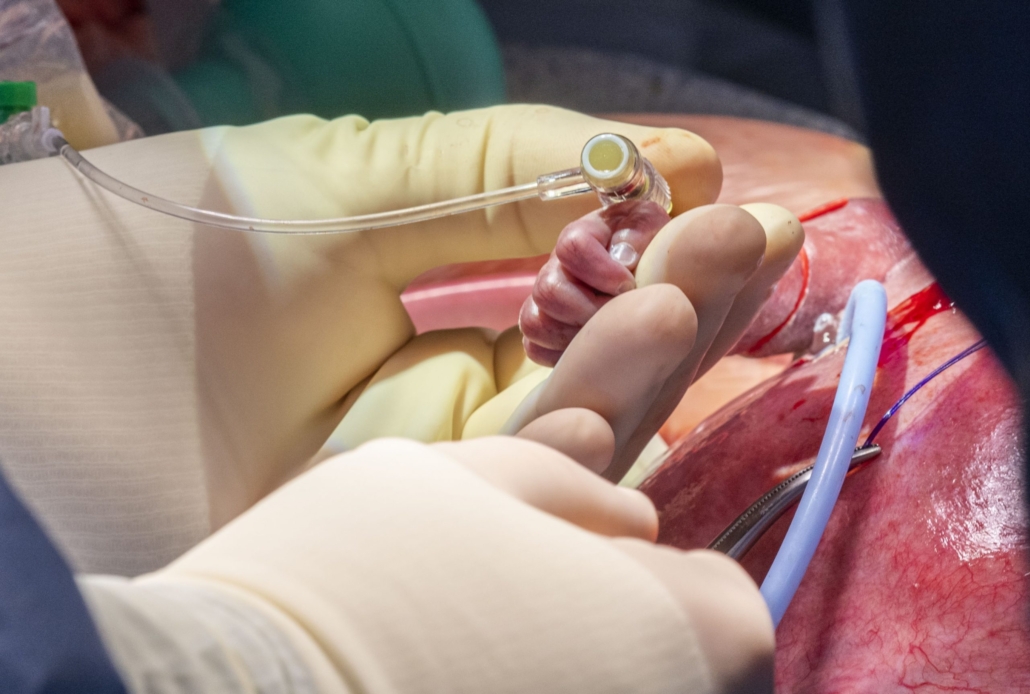
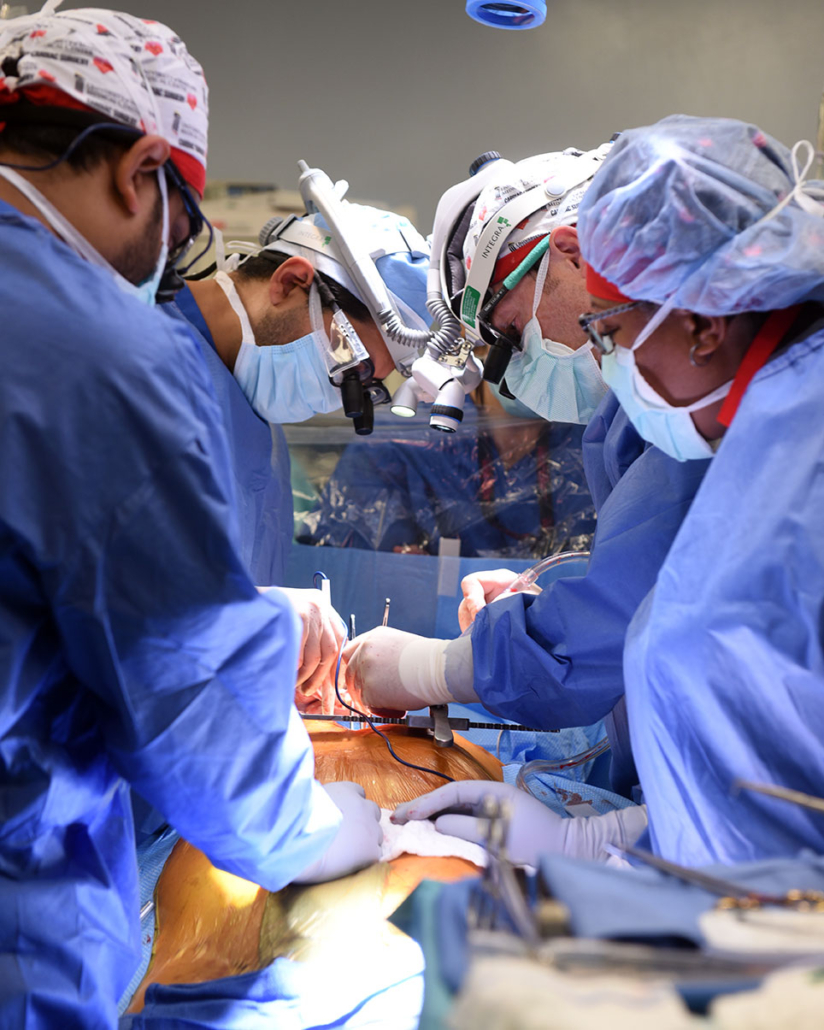
Hani Najm, M.D., who led the heart surgery team, inserts an IV line in the foetus’s right arm to deliver fluids and medications as needed. — courtesy Cleveland Clinic
A multidisciplinary team of Cleveland Clinic and Cleveland Clinic Children’s doctors and nurses performed a rare and complex lifesaving foetal surgery to remove a tumour attached to the heart of a 26-week-old foetus.
Few studies about the rare condition – an intrapericardial teratoma with foetal hydrops (i.e., fluid accumulation) evolving to foetal heart failure – have been reported in medical journals. “Only one previous incidence of continued pregnancy and delivery after foetal intrapericardial teratoma resection is documented in the world’s medical literature,” said Darrell Cass, M.D., director of Cleveland Clinic’s Fetal Surgery and Fetal Care Center. “As far as we know, Cleveland Clinic is the second academic medical centre in the world to have performed this foetal surgery successfully with continued pregnancy and delivery.”
During the procedure in May, surgeons successfully removed the malignant tumour, which had been compressing the left side of the foetus’ heart and impairing circulation, leading to fluid accumulation around the heart and other organs of the foetus.
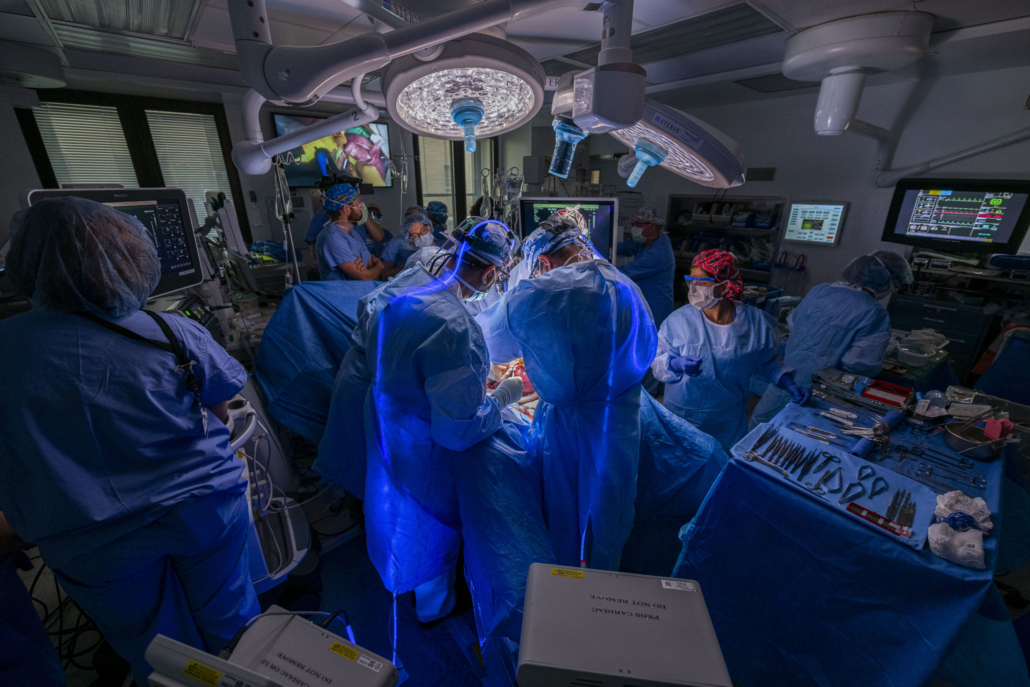
The surgery included paediatric and congenital heart surgeons, a paediatric cardiologist, obstetric and paediatric anaesthesiologists, and a maternal-foetal medicine specialist.
— courtesy Cleveland Clinic
Following the operation, the mother and foetus recovered well with no complications and no sign of tumour recurrence during prenatal check-ups. On July 13, at 36 weeks and two days, a baby boy was delivered near full term by Caesarean section. Both mother and baby are doing well.
“I am very proud of our talented congenital heart surgery and foetal surgery teams that integrated seamlessly to successfully perform a complex lifesaving foetal surgery,” said Dr. Cass. “This tumour was growing rapidly in the exact wrong spot. It was compressing the heart of the foetus, causing fluid accumulation, and we started seeing signs that the cardiac function was deteriorating. We needed to act quickly and decisively to rescue the foetus.”
To perform this foetal surgery, Dr. Cass led a team that included paediatric and congenital heart surgeons Hani Najm, M.D., and Alistair Phillips, M.D.; paediatric cardiologist Francine Erenberg, M.D.; obstetric and paediatric anaesthesiologists McCallum Hoyt, M.D., Tara Hata, M.D., Yael Dahan, M.D.; and maternal-foetal medicine specialist, Amanda Kalan, M.D., who provided the mother’s care, including delivery of the newborn baby boy 10 weeks after the foetal surgery.
See the animation of the foetal intrapericardial teratoma resection
https://youtu.be/v-kOveNbbio
Once the mother was anesthetized with an approach to provide the safest environment for the foetal heart surgery to be successful, a Caesarean section-like incision was made to expose the mother’s uterus. Ultrasound was used to carefully locate the placenta and foetus, and best location for entry. The uterus was then opened about 12 cm, and the arms of the foetus were brought out to expose the chest.
Dr. Najm, who led the heart surgery team, inserted an IV line in a blood vessel of the foetus’s right arm to deliver fluids and medications as needed. Then, he carefully opened the chest and pericardium, and removed the tumour from the beating foetal heart. “As soon as the tumour was removed, the compression of the left atrium disappeared, and there was a nice blood flow that was almost back to normal,” said Dr. Najm.
Following the completion of the foetal heart surgery, the chest of the foetus was closed and the foetus was positioned back in the uterus. The uterus was then closed, followed by closure of the mother’s abdomen. The surgery lasted 3 ½ hours.

Rylan Harrison Drinnon was born on July 13 to Sam and Dave Drinnon.
Both mother and foetus recovered well following the surgery. The foetus’s cardiac function immediately improved, and the foetus stayed in the womb for the remainder of the pregnancy. Ten weeks later, the baby was born.
“Such an innovative foetal surgery provides hope to other families who may receive a similar devastating diagnosis,” said Dr. Najm, chair of Pediatric and Congenital Heart Surgery at Cleveland Clinic Children’s. “Clinical teams from Cleveland Clinic and Cleveland Clinic Children’s are consistently collaborating and remain dedicated to innovation and teamwork to ensure our patients of all ages can feel safe when entrusting their care to us.”
Moving forward, the infant’s healthcare team will monitor his heart health and check that there are no signs of tumour recurrence. In the future, the child will likely need surgery to reposition together his sternum that did not properly heal in utero.
“In this case, time was of the essence,” said Dr. Cass. “Shortly after the patient arrived at Cleveland Clinic, imaging tests showed that the tumour kept growing and the foetus’s heart function was deteriorating. It is important to acknowledge the whole care team. This family’s maternal foetal medicine specialist accurately diagnosed the condition and reached out to us because of our expertise in foetal care and treatment.”
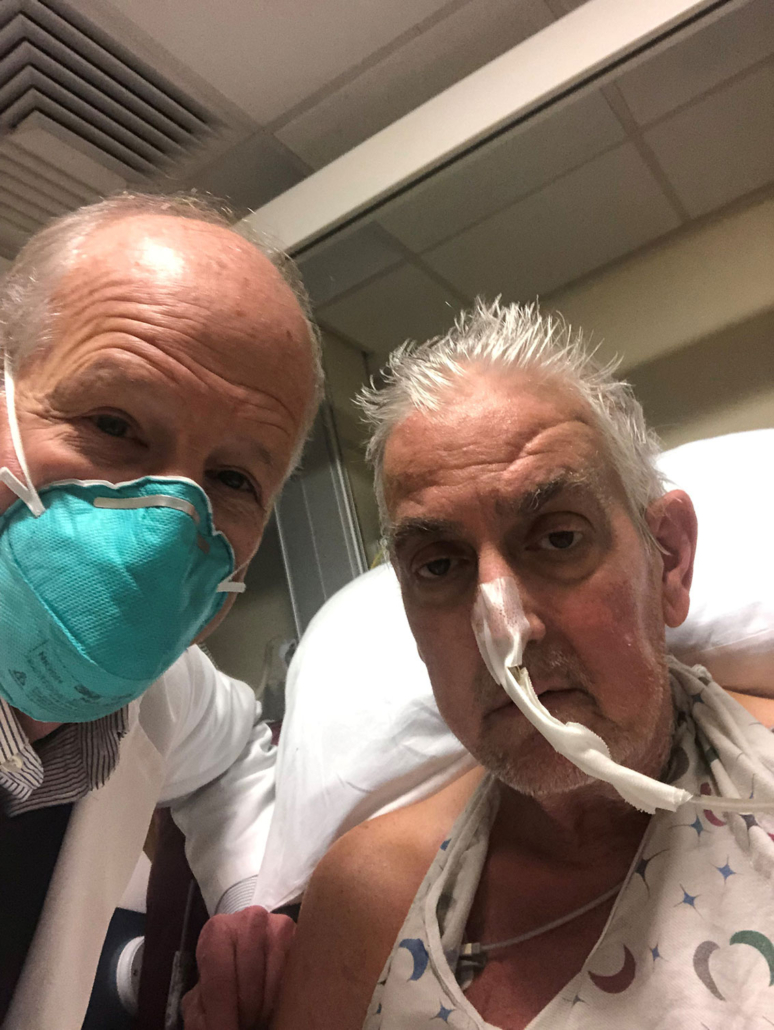
Bartley P. Griffith, MD and the patient, David Bennett — University of Maryland School of Medicine
In a major medical breakthrough, a man in the United States has received a heart from a genetically modified pig in a successful transplant.
The 57-year-old man, David Bennett, who was not eligible for a routine heart transplant, opted for the experimental surgical procedure, saying: “It was either die or do this transplant. I want to live. I know it’s a shot in the dark, but it’s my last choice.”
The eight-hour surgery took place on Friday 7 January and at the time of this publication, the patient was reportedly doing well.
The historic surgery was conducted by a team lead by Bartley P. Griffith, MD, and Muhammad M. Mohiuddin, MD, at the University of Maryland Medicine.
This experimental transplant procedure shows that a genetically-modified animal heart can function like a human heart without immediate rejection by the body and has major implications for the future of transplant surgery.
The U.S. Food and Drug Administration granted emergency authorization for the surgery on New Year’s Eve through its expanded access (compassionate use) provision. It is used when an experimental medical product, in this case the genetically-modified pig’s heart, is the only option available for a patient faced with a serious or life-threatening medical condition.
Dr Griffith, who is the Thomas E. and Alice Marie Hales Distinguished Professor in Transplant Surgery at University of Maryland School of Medicine (UMSOM), commented: “This was a breakthrough surgery and brings us one step closer to solving the organ shortage crisis. There are simply not enough donor human hearts available to meet the long list of potential recipients. We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future.”
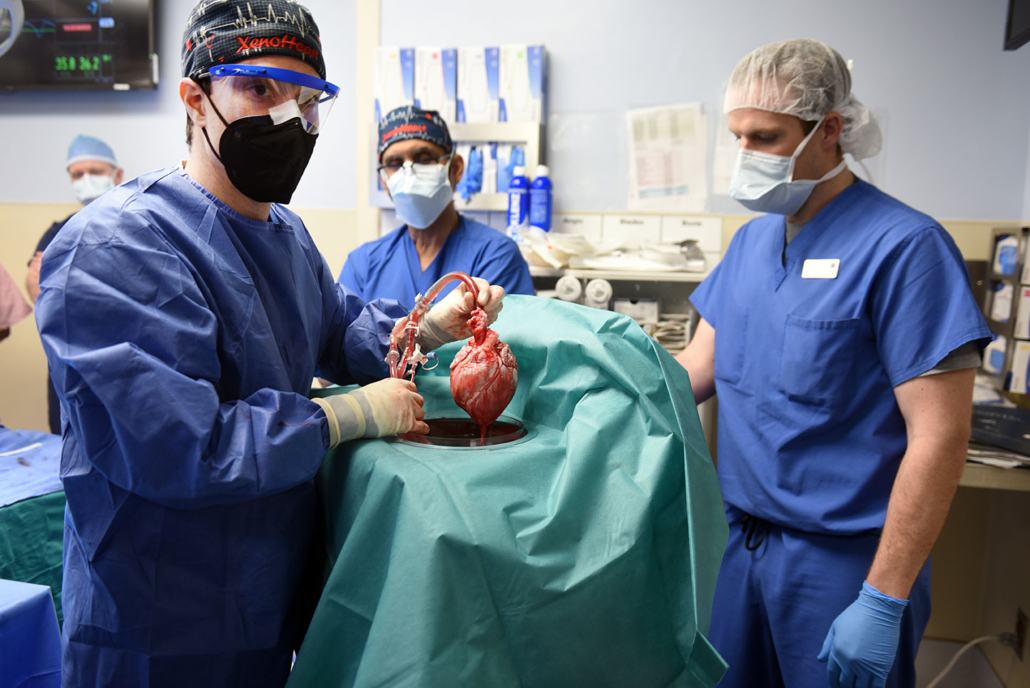
Prior to transplant, the heart from the genetically modified pig is removed from a machine perfusion device to keep it preserved until surgery — University of Maryland School of Medicine
Dr Mohiuddin, Professor of Surgery at UMSOM is considered one of the world’s foremost experts on transplanting animal organs, known as xenotransplantation. He established the Xenotransplantation Program five years ago with Dr. Griffith at UMSOM.
“This is the culmination of years of highly complicated research to hone this technique in animals with survival times that have reached beyond nine months. The FDA used our data and data on the experimental pig to authorize the transplant in an end-stage heart disease patient who had no other treatment options,” Dr Mohiuddin said. “The successful procedure provided valuable information to help the medical community improve this potentially life-saving method in future patients.”
The genetically modified pig was provided by Revivicor, a regenerative medicine company based in Blacksburg, Virginia, US.
Three genes – responsible for rapid antibody-mediated rejection of pig organs by humans – were knocked out in the donor pig. Six human genes responsible for immune acceptance of the pig heart were inserted into the genome. Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue.
The transplant surgical team was lead by Bartley P. Griffith, MD, and Muhammad M. Mohiuddin, MD. — University of Maryland School of Medicine
Dr. Mohiuddin, Dr. Griffith, and their research team have spent the past five years perfecting the surgical technique for transplantation of pig hearts into non-human primates.
“As a cardiothoracic surgeon who does lung transplants, this is an amazing moment in the history of our field. Decades of research here at Maryland and elsewhere have gone into this achievement. This has the potential to revolutionize the field of transplantation by eventually eliminating the organ shortage crisis,” said Christine Lau, MD, MBA the Dr. Robert W. Buxton Professor and Chair of the Department of Surgery at UMSOM and Surgeon-in-Chief at UMMC. “This is a continuation of steps to making xenotransplantation a life-saving reality for patients in need.”
The New York Times quoted Dr. Griffith as saying he first broached the experimental treatment in mid-December. It was a “memorable” and “pretty strange” conversation.
“I said, ‘We can’t give you a human heart; you don’t qualify. But maybe we can use one from an animal, a pig,” Dr. Griffith recalled. “It’s never been done before, but we think we can do it.’”
“I wasn’t sure he was understanding me,” Dr. Griffith added. “Then he said, ‘Well, will I oink?’”
Organs from genetically modified pigs have been the focus of much of the research in xenotransplantation, in part because of physiologic similarities between pigs, human, and nonhuman primates.
UMSOM received $15.7 million sponsored research grant to evaluate Revivicor genetically-modified pig ‘UHearts’ in baboon studies.
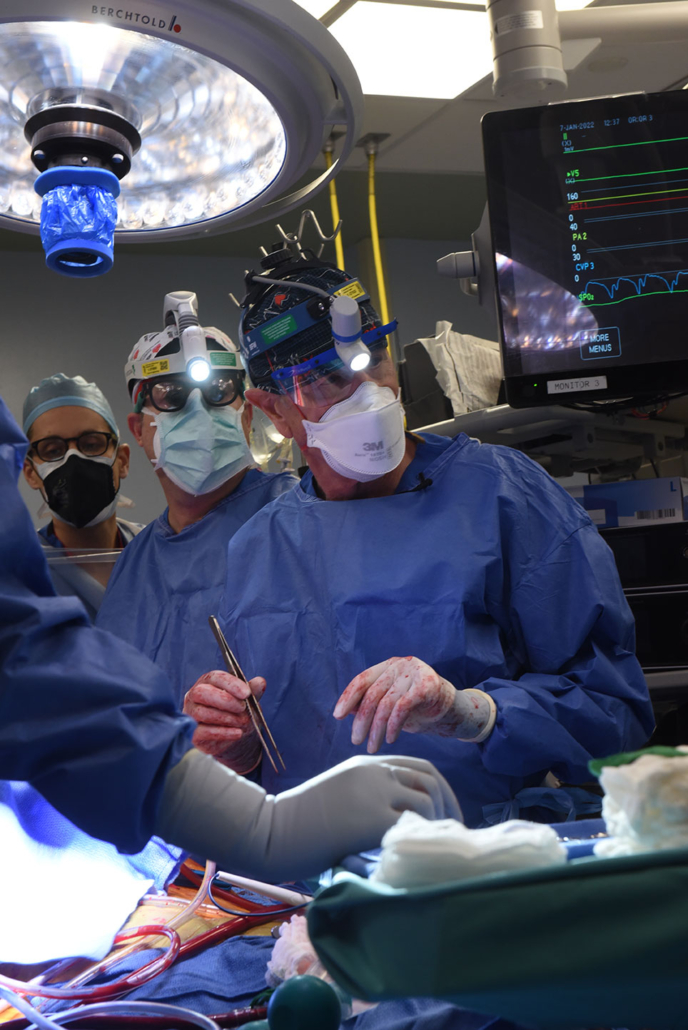
Bartley Griffith, MD, performs the historic transplant — University of Maryland School of Medicine
A new experimental drug made by Kiniksa Pharmaceuticals, along with conventional anti-rejection drugs, which are designed to suppress the immune system and prevent the body from rejecting the foreign organ, were used in the procedure.
Bruce Jarrell, MD, President of the University of Maryland, Baltimore, who himself is a transplant surgeon, recalled: “Dr. Griffith and I began as organ transplant surgeons when it was in its infancy. Back then, it was the dream of every transplant surgeon, myself included, to achieve xenotransplantation and it is now personally gratifying to me to see this long-sought goal clearly in view. It is a spectacular achievement.”
The Feinberg research group at Carnegie Mellon University, along with collaborators in the Netherlands, has developed a dynamic heart model comprised of engineered heart muscle tissue designed to mimic physiologic preloads and afterloads.
Abbott has recently launched in the US their Jot Dx, the company’s latest insertable cardiac monitor (ICM). The Jot Dx ICM gives clinicians and hospitals control of how they manage the flow of information through a unique feature to view either all abnormal heart rhythm data or to simplify which irregular heart rhythms are recorded […]
What if a computer simulation model of the heart and blood vessel could reduce the need for human or animal data in clinical trials, while speeding up product development? Biotronik and their research partners are looking into exactly that question in the new EU-funded SIMCor project. One of the first likely developments from this partnership will be an implantable sensor to better manage heart failure.
More than 10 million people in Europe suffer from heart failure. Beyond its obvious impacts on patient quality of life, treating heart failure uses 1-2% of a developed country’s health budget every year, with two-thirds of that taken up by hospital stays. If we can help reduce hospital visits related to heart failure, we can both help heart failure patients live better lives and reduce overall healthcare costs. That’s just one reason why Biotronik is taking part in SIMCor, a three-year EU-funded project to develop an implantable pressure sensor that aims to help heart failure patients and physicians better manage their condition.
Along with Biotronik, SimCor includes 11 partners from eight countries, including Berlin’s Charité Hospital. By pooling resources and data, SIMCor’s goal is to speed up the development of this technology and achieve results as fast as possible.
Beyond a new and innovative technology to support heart failure patients, the SIMCor partnership also has the potential to provide even longer-lasting benefits. If successful, computer simulations of the heart-implant interaction could speed up product testing and regulatory approval, providing many patients with technology that can save and improve their lives in a more timely manner.
The SIMCor project focuses on developing computer simulation technology that can help test and validate medical devices. These computerized tests could replace the need for animal testing and help make clinical studies even safer for patients. If a large and high-quality dataset is available, researchers can simulate clinical interventions in virtual patient cohorts. Over the longer term, this could reduce clinical trial size by 25%, with 30% less time required to complete studies. In the end, this allows medical devices to be quickly approved to help patients. The US FDA has already noted the potential positive effects such modelling could have, and is encouraging the development of simulation technology. By working together, Biotronik and its SIMCor partners can conduct these simulations using far bigger datasets than would otherwise be available, yielding the sophisticated modelling required to simulate heart and blood vessels.

Dr. Torsten Luther, Director of Product Development for Delivery Tools, Leads & Accessories in R&D at Biotronik
“We need to demonstrate that implants perform well across the whole patient population. That’s a long and sometimes challenging process because patient anatomy can vary widely, especially due to diseases,” said Dr. Torsten Luther, Director of Product Development for Delivery Tools, Leads & Accessories in R&D at Biotronik. “Using a large data pool to simulate different parts of the cardiovascular system, such as the heart or pulmonary artery, allows us to test implant performance across a wide range of anatomies representing the whole patient population. We can then optimize our technology for everyone.”
Research and development have always been a priority at Biotronik. Since developing the first German pacemaker in 1963, Biotronik has continued to pave the way for pioneering innovations. In its Berlin headquarters alone, one out of every five employees work in R&D, ensuring that medical technology keeps pace with the interests and needs of future patients and physicians.
By investing in clinical trials and initiating research projects, Biotronik seeks to address research gaps and offer practical treatment options.

Dr. Andreas Arndt, Team Lead R&D Sensors and SIMCor project coordinator at Biotronik
“The SimCor project is a great example of how we work together with like-minded partners from different industries as well as academic institutions across Europe. We profit from each other’s knowledge and together, we can make an impactful contribution to medical research. In this regard, I believe that collaboration can be a key driver for innovation,” said Dr. Andreas Arndt, Team Lead R&D Sensors and SIMCor project coordinator at Biotronik.
YouTube: https://youtu.be/8YMsoXsH4P8
Robocath’s R-One robotic platform has been used to successfully complete the first five robotic coronary angioplasties in Belgium.
The first-in-human use of Foldax’s biopolymer Tria™ heart valve as a replacement for a diseased mitral valve has shown positive results. The first case was performed by David Heimansohn, MD, at Ascension St. Vincent Hospital, Indianapolis, as part of a U.S. early feasibility research study.
Biotech scientists have developed a new polymeric heart valve, called the PoliValve, with a life span potentially longer than current artificial valves.
The PoliValve will also prevent the need for the millions of patients with diseased heart valves to require life-long blood thinning medication.
There are two artificial valves currently available for patients with diseased heart valves; both have limitations either in durability or in biocompatibility. Biological valves are made from fixed pig or cow tissue and have good biocompatibility, but limitations in durability of 10 to 15 years. Mechanical valves have very good durability, but poor biocompatibility and patients must take daily blood thinning drugs to prevent blood clots.
The PoliValve, created by Prof. Geoff Moggridge, Dr Marta Serrani and Dr Joanna Stasiak at Cambridge’s Department of Chemical Engineering and Biotechnology, and Prof. Raimondo Ascione, Head of the Translational Biomedical Research Centre (TBRC) at the University of Bristol, is made from a special co-polymer and is designed to resemble the flexibility, biocompatibility and durability of a natural heart valve. They have spent three years conducting developmental work and extra-vivo and in-vivo testing on the new PoliValve.
The device combines excellent durability with biocompatibility, addressing the limitations of current biological and mechanical artificial valves. It is made through a simple moulding process; hence it also reduces markedly manufacture and quality control costs.
Initial testing in animal has been undertaken at Bristol’s TBRC facility as a first mandatory in-vivo testing step to ensure safety. Long-term in-vivo testing is already planned and funded as a necessary additional step before bringing it to market.
According to the ISO standards a new artificial heart valve must withstand a minimum of 200 million repetitions of opening and closing during bench testing (equivalent to five-year life span) to be tested in humans. The new Cambridge- Bristol polymeric valve has comfortably surpassed this.
Prof. Ascione noted: “The transformational PoliValve results from an advanced Bristol/ Cambridge-based biomedical cross-fertilisation between experts in biomaterials, computational modelling, advanced preclinical development/ testing and clinical academics understanding the patient needs. The new valve could help millions of people worldwide and we aim to test in patients within the next five years.”
Read more
April 2024
The medical devices information portal connecting healthcare professionals to global vendors
Prins Hendrikstraat 1
5611HH Eindhoven
The Netherlands
info@interhospi.com
PanGlobal Media IS not responsible for any error or omission that might occur in the electronic display of product or company data.
This site uses cookies. By continuing to browse the site, you are agreeing to our use of cookies.
Accept settingsHide notification onlyCookie settingsWe may ask you to place cookies on your device. We use cookies to let us know when you visit our websites, how you interact with us, to enrich your user experience and to customise your relationship with our website.
Click on the different sections for more information. You can also change some of your preferences. Please note that blocking some types of cookies may affect your experience on our websites and the services we can provide.
These cookies are strictly necessary to provide you with services available through our website and to use some of its features.
Because these cookies are strictly necessary to provide the website, refusing them will affect the functioning of our site. You can always block or delete cookies by changing your browser settings and block all cookies on this website forcibly. But this will always ask you to accept/refuse cookies when you visit our site again.
We fully respect if you want to refuse cookies, but to avoid asking you each time again to kindly allow us to store a cookie for that purpose. You are always free to unsubscribe or other cookies to get a better experience. If you refuse cookies, we will delete all cookies set in our domain.
We provide you with a list of cookies stored on your computer in our domain, so that you can check what we have stored. For security reasons, we cannot display or modify cookies from other domains. You can check these in your browser's security settings.
.These cookies collect information that is used in aggregate form to help us understand how our website is used or how effective our marketing campaigns are, or to help us customise our website and application for you to improve your experience.
If you do not want us to track your visit to our site, you can disable this in your browser here:
.
We also use various external services such as Google Webfonts, Google Maps and external video providers. Since these providers may collect personal data such as your IP address, you can block them here. Please note that this may significantly reduce the functionality and appearance of our site. Changes will only be effective once you reload the page
Google Webfont Settings:
Google Maps Settings:
Google reCaptcha settings:
Vimeo and Youtube videos embedding:
.U kunt meer lezen over onze cookies en privacy-instellingen op onze Privacybeleid-pagina.
Privacy policy