New study sheds light on cellular vulnerabilities and resilience in Alzheimer’s disease
A groundbreaking MIT study published in Nature offers new insights into the cellular mechanisms underlying Alzheimer’s disease, revealing potential targets for interventions to maintain cognitive function.
Researchers at the Massachusetts Institute of Technology (MIT) have made significant strides in understanding the cellular and molecular underpinnings of Alzheimer’s disease. The study, published 24 July 2024 in Nature [1], provides a comprehensive analysis of gene expression patterns across multiple brain regions in individuals with and without Alzheimer’s disease.
The research team, led by Li-Huei Tsai and Manolis Kellis, employed single-cell RNA profiling to examine more than 1.3 million cells of over 70 cell types in six brain regions. This unprecedented level of detail allowed the researchers to identify specific cell types and circuits that become vulnerable in Alzheimer’s disease, as well as factors that may contribute to cognitive resilience in some individuals.
“Specific brain regions are vulnerable in Alzheimer’s and there is an important need to understand how these regions or particular cell types are vulnerable,” said Li-Huei Tsai, Picower Professor of Neuroscience and director of The Picower Institute for Learning and Memory and the Aging Brain Initiative at MIT.
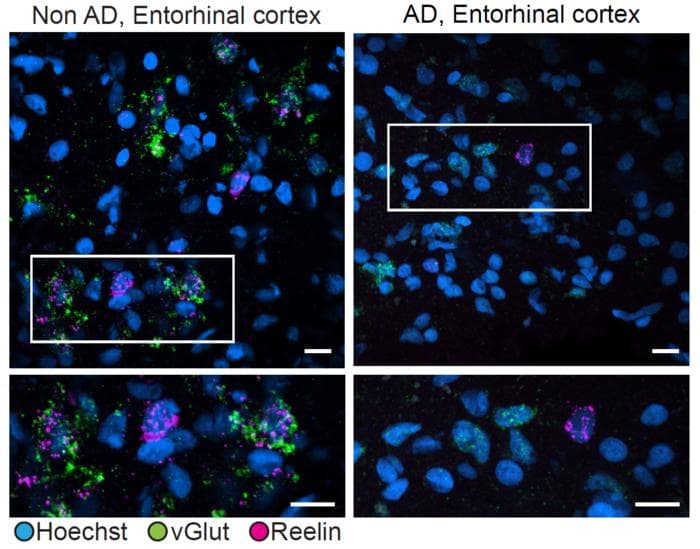
Researchers compared Reelin expression in excitatory neurons in the entorhinal cortex of people with (right) or without (left) Alzheimer’s disease. In people without the disease, vGlut (green), a marker of excitatory neurons, and Reelin (magenta) were often expressed together. In people with Alzheimer’s, excitatory cells exhibited much less Reelin expression.
The Reelin connection
One of the study’s key findings centres on the protein Reelin and its potential role in Alzheimer’s disease progression. The researchers identified specific types of excitatory neurons in the hippocampus and entorhinal cortex that were significantly reduced in individuals with Alzheimer’s. These neurons were found to either directly express Reelin or be affected by Reelin signalling.
The importance of Reelin in Alzheimer’s research has been highlighted by a recent case study of a man in Colombia who remained cognitively healthy despite a strong genetic predisposition to early-onset Alzheimer’s. This individual possessed a rare mutation that increased Reelin activity, suggesting a potential protective effect.
“We can think of Reelin as having maybe some kind of protective or beneficial effect,” said Leyla Akay, a graduate student in Tsai’s lab. “But we don’t yet know what it does or how it could confer resilience.”
Astrocytes and cognitive resilience
In addition to identifying vulnerable cell populations, the study also explored factors associated with cognitive resilience in the face of Alzheimer’s pathology. The researchers found that astrocytes expressing genes related to antioxidant activity, choline metabolism, and polyamine biosynthesis were significantly associated with sustained cognitive function, even in the presence of high levels of tau and amyloid proteins.
These findings align with previous research by Tsai and colleagues, which demonstrated that dietary choline supplementation could help astrocytes cope with lipid dysregulation caused by the APOE4 gene variant, a significant risk factor for Alzheimer’s disease.
Methodology and data sharing
To analyse the vast amount of single-cell data collected, the research team developed a novel methodology based on groups of coordinately expressed genes, known as “gene modules”. This approach allowed for a more robust analysis of gene expression patterns across cell types and brain regions.
“In principle, the 1.3 million cells we surveyed could use their 20,000 genes in an astronomical number of different combinations,” explained Kellis. “In practice, however, we observe a much smaller subset of coordinated changes. Recognising these coordinated patterns allows us to infer much more robust changes, because they are based on multiple genes in the same functionally-connected module.”
Future directions
The researchers have made their extensive dataset and analytical tools publicly available, hoping to facilitate further discoveries in the field of Alzheimer’s research. Kellis and his team are now focusing on studying the control circuitry associated with the differentially expressed genes to understand the genetic variants, regulators, and other factors that could potentially be modulated to reverse disease progression across brain regions, cell types, and different stages of Alzheimer’s disease.
This groundbreaking study not only provides valuable insights into the cellular mechanisms underlying Alzheimer’s disease but also offers potential targets for future therapeutic interventions. By identifying specific cell types and molecular pathways associated with both vulnerability and resilience, the research paves the way for more targeted approaches to maintaining cognitive function in individuals at risk of or living with Alzheimer’s disease.
Reference:
- Mathys, H., Boix, C., Akay, L., al. (2024). Single-cell multiregion dissection of Alzheimer’s disease. Nature. https://doi.org/10.1038/s41586-024-07606-7

