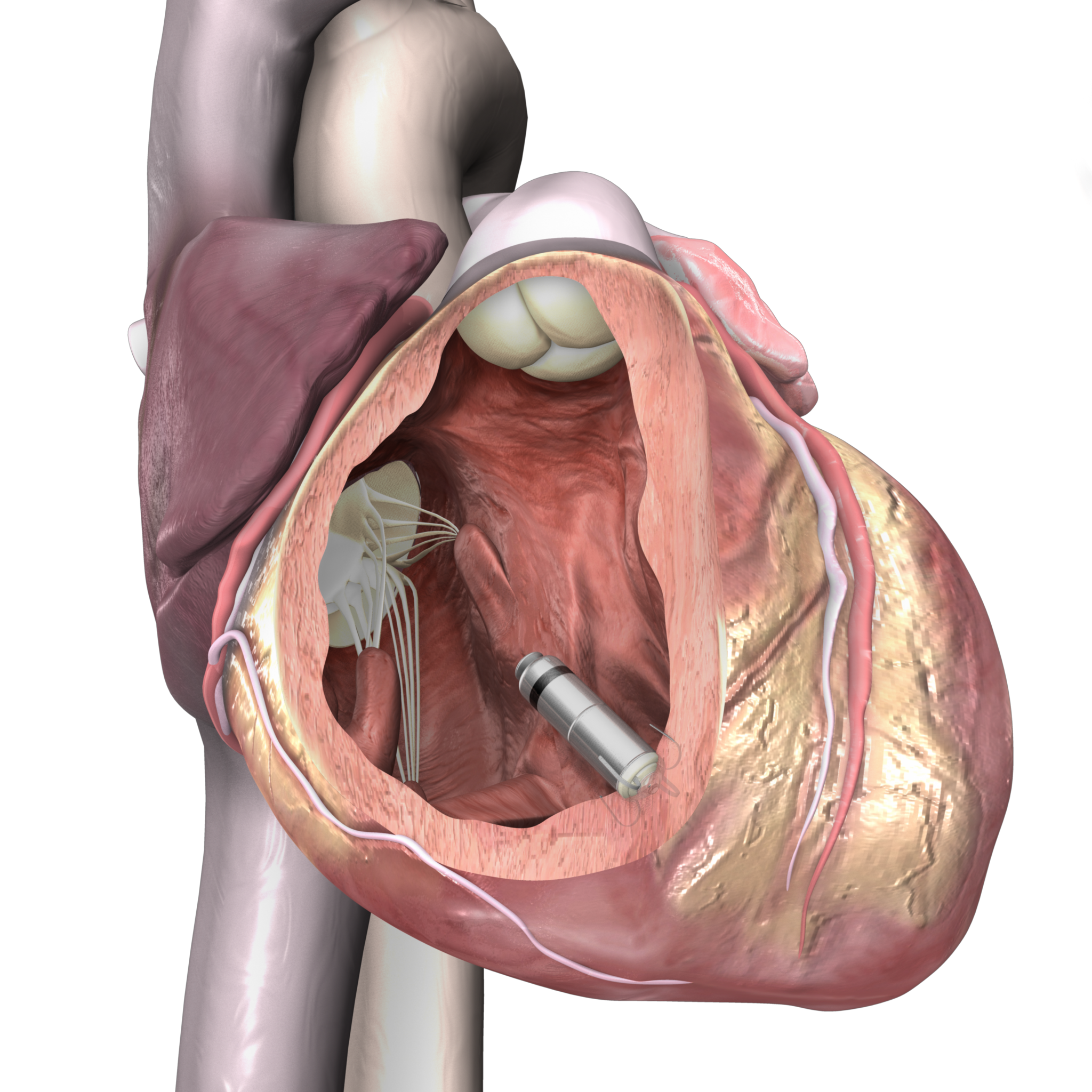
Medtronic receives CE Mark for NextGen miniature Micra leadless pacemakers
Medtronic has received the CE Mark for its Micra AV2 and Micra VR2 miniature, leadless pacemakers.
Micra AV2 and Micra VR2, the world’s smallest pacemakers, provide longer battery life and easier programming than prior Micra pacemakers, while still delivering the many benefits of leadless pacing such as reduced complications compared to traditional pacemakers.
With approximately 40% more battery life compared to previous generations, Medtronic projects the battery life of Micra AV2 and Micra VR2 is nearly 16 and 17 years, respectively. This means more
than 80% of patients who receive a Micra may only require one device for life.
The US FDA approved the Micra AV2 and VR2 devices in 2023.
In addition to size and longevity benefits, Micra devices are also the only leadless pacemakers with remote monitoring capabilities.
“For more than eight years, our Micra leadless pacemakers have provided meaningful benefits to people in Europe who require a pacemaker. Now, these patients have access to the latest leadless pacing technology that, for most of them, may be the only device they will ever need,” said Robert C. Kowal, M.D., Ph.D., general manager, Cardiac Pacing Therapies within the Cardiac Rhythm Management business, which is part of the Cardiovascular Portfolio at Medtronic.
“The first generation of leadless Micra pacemakers demonstrated a significant reduction in major complications, and this next generation of Micra brings several additional benefits including greater longevity, and specifically for the Micra AV2, new algorithms to optimize AV synchrony at faster heart rates while requiring less in-office programming,” said Prof. Dr Christophe Garweg, Cardiologist at University Hospitals of Leuven, Belgium.”
Globally, more than 200,000 patients have received Micra pacemakers. Comparable in size to a large vitamin capsule, Micra pacemakers are less than one-tenth the size of traditional pacemakers. Unlike traditional pacemakers, Micra pacemakers do not require leads or a surgical “pocket” under the skin, so potential sources of complications related to leads and pockets are eliminated, and there is no visible sign of the device.
For more information, visit: www.Medtronic.com
Digital issue: Please click here for more information