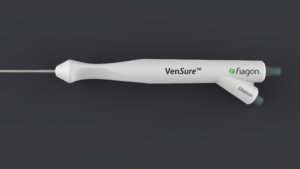
Intersect ENT’s VenSure Balloon Sinus Dilation System and Cube Navigation System now available in US
Intersect ENT, a global ear, nose and throat medical technology company, says their VenSure Balloon Sinus Dilation System and Cube 4D Navigation System with VirtuEye photo registration is now commercially available in the United States.
The VenSure Balloon and Cube 4D Navigation Systems are used in procedures that are designed to improve debilitating chronic rhinosinusitis (CRS) symptoms. VirtuEye photo registration is a novel, touchless technology that allows for easy 3D facial registration, pinpoint accuracy and improved workflow efficiency for balloon sinus dilation (BSD) procedures and other ENT related skull-based surgeries.
With the addition of the Cube and VenSure technologies to the portfolio of localized drug delivery products, PROPEL (mometasone furoate) and SINUVA (mometasone furoate) sinus implants, the company now offers a more comprehensive and integrated set of treatment solutions.
“Chronic rhinosinusitis is an underserved and serious condition that can reduce patients’ quality of life, their ability to work and to get restful sleep. There is a clear need for innovative tools and a broadened armamentarium that can help physicians treat patients suffering from CRS,” said Rajiv Pandit, MD, Otolaryngologist at Dallas ENT Head & Neck Surgery. “Balloon sinus dilation offers CRS patients a convenient, less-invasive, lower-cost solution by providing rapid resolution of symptoms with minimal risk of complications. The VenSure Balloon allows the technique to be tailored and adapted to each patient’s unique anatomy and the use of Cube Navigation System with VirtuEye ensures a simple yet highly precise location of the balloon for optimized placement within the sinus.”
The BSD procedure entails insertion of a balloon, like the VenSure device, to open the blocked sinuses with precision, reducing the risk of complications associated with other, more invasive surgical procedures. Because BSD preserves the integrity of the sinus tissue, it allows for quick recovery times. Additionally, the sinus procedure can be performed in the physician’s office. The VenSure Sinus Balloon is a simple yet effective device with a slim, ergonomic design that tailors to the user’s hand for efficient manoeuvrability. The systems responsive feel is driven by its malleability and integrated components intended to optimize visibility and feel. The tip tracked technology that is incorporated into each instrument helps provide confident and precise placement feedback.
Accompanying the VenSure Sinus Balloon introduction is the Cube 4D Navigation system, a next generation electromagnetic navigation system that is intuitive, simple and precise. The Cube’s compact design and small footprint easily integrates into any cart or tower shelf. The systems intuitive interface with on-screen instructions and audible confirmation tones, as well as an open architecture platform allowing flexibility to track preferred instrumentation, provides simplicity of use for physicians.
Exclusive to the Cube 4D system is the innovative and touchless VirtuEye photo registration technology that reduces patient registration time to under 40 seconds, and its ease of use enhances the user experience and improves pre-procedure efficiency. Importantly, VirtuEye is designed to collect over 50,000 patient registration points in one camera shot, which mitigates common tactile tracing errors that can occur with most other registration approaches. As a touchless technology, VirtuEye photo registration also helps to avoid breaking the sterile field by reducing direct interaction between the care team and the patient.
The VenSure Nav Balloon Device is intended for use in conjunction with the Cube Navigation System during sinus procedures when surgical navigation or image-guided surgery may be necessary to locate and move tissue, bone or cartilaginous tissue surrounding the drainage pathways of frontal, maxillary, and sphenoid sinuses to facilitate dilation of the sinus ostia.