Canon Medical unveils AI automation platform for diagnostic imaging
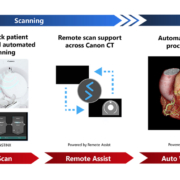
Canon Medical Systems has introduced an artificial intelligence-powered automation platform designed to enhance diagnostic workflows and expedite clinical decision-making in emergency care settings. The zero-click solution integrates deep learning technology to streamline image analysis across multiple imaging modalities.






 © Cheng-Hui Li and Pengfei Zheng
© Cheng-Hui Li and Pengfei Zheng