Medtronic receives CE Mark for Micra AV TPS
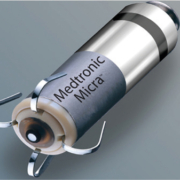
Medtronic has received CE Mark for Micra™ AV Transcatheter Pacing System (TPS), the world’s smallest pacemaker with atrioventricular (AV) synchrony. Micra AV is indicated for the treatment of patients with AV block, a condition in which the electrical signals between the chambers of the heart (the atria and the ventricle) are impaired. Medtronic now offers the only CE Mark approved leadless pacemaker portfolio. First implants of Micra AV recently occurred in Spain by Dr. José Ramón González Juanatey at the University Hospital in Santiago de Compostela.
“This new device not only stimulates but is also able to recognize the electrical activity of the whole heart. Now we can extend this wireless technology to our patients who require dual chamber stimulation and in whom traditional stimulation cannot be performed or is conditioned by previous infections, occlusions of the vessels of the upper extremities, etc.,” said Dr. Juanatey, director, Cardiology and Intensive Cardiac Care Department, full professor of Cardiology, University Hospital, Santiago de Compostela, Spain (past-president, Spanish Society of Cardiology). Historically, patients with AV block have been treated with traditional dual-chamber pacemakers which are implanted in the upper chest, under the skin below the collar bone, and connected to the heart using thin wires called “leads”. Identical in size and shape to the original Micra TPS, Micra AV has several additional internal atrial sensing algorithms which detect cardiac movement, allowing the device to adjust pacing in the ventricle to coordinate with the atrium, providing “AV synchronous” pacing therapy to patients with AV block.
Unlike traditional pacemakers, Micra does not require leads or a surgical “pocket” under the skin, so potential sources of complications related to leads and pockets are eliminated – as are any visible signs of the device.
For more information, visit: www.medtronic.com
Read more