Mortara Instrument, Inc. Announces Expansion of ECG Warehouse Contract Award with the U.S. FDA
Mortara has been awarded a multi-year contract for ongoing maintenance and support of the FDA ECG Warehouse including continuous ECG studies analyzed by VERITASTM.
Mortara collaborated with the FDA to develop the ECG Warehouse which was initially deployed in 2005. The ECG Warehouse acts as a repository for annotated electrocardiograph (‘ECG’) studies provided to the FDA in support of new drug applications. With the ECG Warehouse, the FDA uses Mortara’s VERITAS ECG algorithms and viewing technologies to review ECG data submitted as part of new drug applications.
Since inception of the ECG Warehouse, more than 9 million resting ECGs have been analyzed with Mortara’s VERITAS algorithms, making this one of the largest cloud-based clinical data repositories in the world. The ECG Warehouse has subsequently been expanded to also include continuous 12-lead recordings, which now number nearly 800 in total. The warehouse tools include web-based upload, navigation of continuous data, arrhythmia identification and waveform morphology comparison.
Under this expanded ECG Warehouse contract, Mortara will continue to support Sponsor and ECG Central Laboratory upload of ECG studies, provide support to FDA personnel and provide on-going basic development enhancements to the ECG Warehouse including advances in the VERITAS ECG algorithms.
‘Mortara is pleased to continue its longstanding relationship with the FDA in providing the ECG Warehouse solution,’ said Dr. Justin Mortara, CEO of Mortara. ‘This award is testimony to our leadership role in ECG acquisition and algorithm technologies. We are honored to be chosen by the FDA and to play our part in the cardiac safety evaluation of new drugs.’
About Mortara
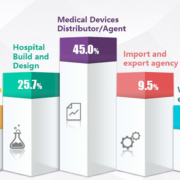
For over 30 years, Mortara Instrument, Inc. has served as a leading designer, developer, and manufacturer of diagnostic cardiology and, most recently, patient monitoring technologies. Mortara is focused on delivering world-class medical devices, as evidenced by its innovative portfolio of solutions designed to serve throughout the continuum of clinical care. The company’s comprehensive range of products spans modalities including resting ECG, cardiac stress exercise, Holter monitoring, cardiac and pulmonary rehabilitation, and ambulatory blood pressure and multi-parameter patient monitoring. Mortara’s global headquarters is located in Milwaukee, Wisconsin with direct operations in Australia, Germany, Italy, the Netherlands, and the United Kingdom. While Mortara distributes its products and technologies globally, it remains dedicated to manufacturing in the United States in order to consistently deliver the quality products for which it is known.
Mortara’s approach to innovation has a global reach that impacts both mature and emerging healthcare systems. To learn more about Mortara and its expanding product portfolio, including the Burdick and Quinton brands, visit www.mortara.com.