High-speed communications and healthcare – Tele-ICUs and ambulance telemedicine
The communications revolution has opened up dramatic possibilities for healthcare delivery across physical/geographical boundaries. Telemedicine, once held up as a miracle, and then seemingly forgotten, has been making a comeback in one of the most challenging frontiers of modern medicine – the ICU.
High-speed communications have also paved the way for ambulance-based telemedicine.
ICU Telemedicine
Video observation and advanced algorithms
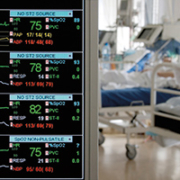
The greatest benefit of ICU telemedicine or Tele-ICU care involves continuous surveillance and interactive care by offsite clinicians. This is achieved by direct video observation of the patient and interrogation of ICU equipment. Advanced computer algorithms, based on clinical data available in a patient