CardioRenal raises €3.3 million to pursue regulatory approval
French company CardioRenal, which specializes in improving treatment at home for patients with severe chronic kidney disease, has secured €3.3 million from business angels and longstanding company shareholders as part of a round of seed capital financing.
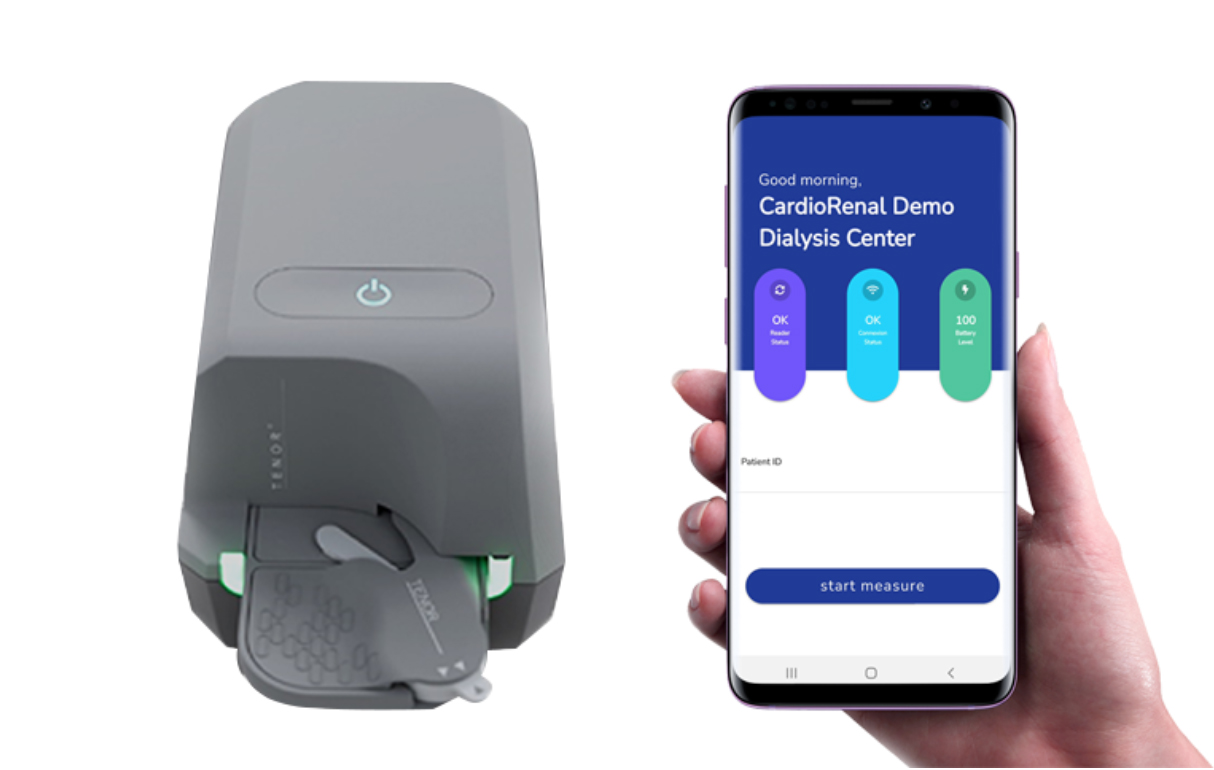
CardioRenal’s mission is to improve chronic cardiovascular disease care for patients at home. Its platform enables doctors to optimize patient treatment through remote monitoring of key blood biomarkers.
This funding will enable the company to pursue its plan to achieve clinical and regulatory approval in preparation for the CE marking of its integrated tool, which is expected in 2023. CardioRenal completed a clinical trial on the technology in October 2021. The results of this trial will be presented at the Heart Failure 2022 congress in Madrid, Spain, in May.
“This fundraising will support our roadmap for our forthcoming launch in Europe and the United States,” said Maurice Bérenger, CEO of CardioRenal.
Globally, around 700 million people live with CKD [1], with many more likely still undiagnosed. These patients frequently have fluctuating blood potassium levels, which exposes them to possible cardiac complications. The associated cardio-vascular events account for up to 30% of deaths in patients with chronic kidney disease [2].
For more information, visit: https://www.cardio-renal.com
References:
1. https://www.thelancet.com/article/S0140-6736(20)30045-3/fulltext
2. https://pubmed.ncbi.nlm.nih.gov/25733525/
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Nulla vulputate condimentum eros. Sed quis luctus leo. Pellentesque pellentesque magna justo, vel ornare purus convallis id. Nunc a libero in sem vehicula pulvinar gravida mattis sem.