Automation is key to saving lives
Personalized tumour treatments are expensive. This particularly applies to individual cancer therapies based on gene-modified T cells. Currently, they are produced manually or, at best, in a partially automated process. In a collaboration project “ProCell for Patient” two hospitals are currently working with Optima Pharma to develop an automated unit for the decentralized production of a CAR-T cell product in treatment centres. This should significantly reduce the time and costs incurred in future manufacturing of those therapies. Jan Deininger and Dr. Ulla Reutner, technical journalist, report.
Every year, more than 430,000 people are diagnosed with leukaemia. Another 500,000 get sick with Non-Hodgkin’s lymphoma [1]. These include forms of the disease where chemotherapy and donating stem cells are unsuccessful.
“Around a quarter of patients who have already undergone treatment can benefit from CAR-T cell therapy,” says Prof. Dr. med. Michael Schmitt, MHBA, Head of GMP Core Facility at the Universitätsklinikum Heidelberg (UKHD), and an expert in cellular immunotherapy. T-cells are genetically modified UKHD. “We modify the cells so that they can subsequently attack cancer cells in the form of what are called killer cells.”
“ The automation of CAR-T cell therapy production is long overdue. Not only for reasons of cost and quality. In the future, it will make the innovation process easier.” Prof. Dr. Walter E. Aulitzky, Chief Physician, Department of Oncology, Hematology and Palliative Medicine, Robert-Bosch-Krankenhaus, Stuttgart.
To do this, the cells are given a kind of “prehensile arm” that enables them to recognize the cancer cells. This is known as a chimeric antigen receptor (CAR). Using viral gene transfer vectors, the genetic information for the CAR is transferred ex vivo to the patient’s T cells. The patient then receives back the CAR-T cells. They multiply within the patient and fight the cancer cells.
Centralized manufacturing is slowing progress
Since 2017, five CAR-T cell products have been approved worldwide: Kymriah (Novartis), Yescarta and Tecartus (Kite/ Gilead), and most recently Breyanzi (BMS) and Abecma (bluebird bio & BMS). There are around 1,220 further cell and gene therapies in the clinical pipeline. Several thousand patients could benefit from this treatment every year. However, the complex industrial production of CAR-T cells currently takes place at just a few pharmaceutical industry sites worldwide, or at what are known as CDMOs (Contract Development and Manufacturing Organizations). The logistics involved and the largely manual production in class A or B clean rooms generate high costs. Meanwhile, there are many university hospitals that already have the expertise to produce CAR-T cell therapies, but their manufacturing processes originate in research, and they are therefore manual and individualized. Standardized and automated manufacturing at these clinics would lead to significant improvements in patient care.
CAR-T cell therapeutics are currently produced manually in the GMP laboratory at Heidelberg University. This is extremely labour-intensive and has to be done in clean room environments at the highest Class A or B levels. Credit: Universitätsklinikum Heidelberg
It is therefore not surprising that the UKHD was the partner of choice for the strategic partnership between Robert-Bosch- Krankenhaus (RBK), Stuttgart, and technology and systems provider Optima Pharma when they were considering developing an automated production unit for CAR-T cell products. As part of a previous project with Charité Hospital in Berlin, Optima Pharma had already taken the first steps towards developing a suitable production platform.
The RBK or its research unit, the Robert Bosch Society for Medical Research, has taken over management of the project. Prof. Dr. Walter E. Aulitzky, Chief Physician of the Department of Oncology, Hematology and Palliative Medicine at RBK is extremely enthusiastic by the opportunities brought by CAR-T cell therapy, but when he thinks about the ways they’ve been made to date, he becomes impassioned: “When you have such a complex process with hundreds of different steps, you can’t really have medical technicians filling pots on a “four-eyes” basis. Automation is long overdue — not just for cost reasons. There is also a quality aspect. In the future, it will also foster innovation, because clinics that are involved in decentralized manufacturing can also contribute to optimizing and developing new cell therapies.”
The first fully automated prototype plant for producing CAR-T cell therapeutics using technology supplied by Optima Pharma will be installed in the GMP facility at the RBK. What was missing so far was the special expertise needed for manufacturing CAR-T cell products.
“It quickly became clear to us that we had to recruit the Universitätsklinikum Heidelberg as a professional manufacturer and Prof. Schmitt as a specialist who knew the process inside out,” recalls Prof. Aulitzky. This was not hard to do, because Prof. Schmitt is also anticipating significant improvements as a result of automation. “The workload will probably be reduced by at least 50 percent. Automated processes can also operate outside of regular working hours.”
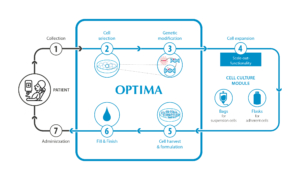
In the Stuttgart/Heidelberg ProCell for Patient model, essential steps (blue) in the production of cell therapeutics are performed in an isolator in a fully automated process. The prototype will be installed at the Robert-Bosch-Krankenhaus in Stuttgart. Credit: Optima
“ With the help of the ProCell for Patient system, it will probably be possible to reduce the amount of work, i.e. the number of hours that qualified staff are spending today on the production of CAR-T cells, by at least 50 percent.” Univ.-Prof. Dr. med. Michael Schmitt, MHBA, Siebeneicher-Endowment Professor for Cellular Immunotherapy, Head of GMP Core Facility, Universitätsklinikum Heidelberg.
“ProCell for Patient” at the heart of a future reference centre
The “ProCell for Patient” project started in October 2020, and is funded by the Ministry of Economic Affairs, Labour and Housing Baden-Württemberg as part of the “Forum Health Region Baden-Wuerttemberg” initiative. From Optima Pharma, Dr. Andrea Traube, who heads up Market Development with a focus on system solutions for cell and gene therapeutics at Optima Pharma, is accompanying the project. As Dr. Traube says: “The first steps have been mastered. These are the detailed process analysis of the CAR-T process in Heidelberg and the creation of the User Requirement Specification (URS).”
Her expertise in automating cell culture processes, according to Prof. Aulitzky, is to ensure “that we not only map the manual process 1:1, but also make potential adjustments to the process to exploit the full potential of automation to optimize the manufacturing process”. Optima has unique expertise in this area.
There are six main steps to the process: cell selection, cell activation, genetic modification to CAR-T cells, cell expansion, cell harvesting and formulation, and fill & finish. By using isolator technology from Optima, the plant does not have to be installed in the highest clean room class, as is the case in the Heidelberg GMP laboratory, but in class C or D clean rooms. The first challenges were already overcome during the preparation of the URS. Prof. Schmitt remembers: “To translate the manual process into a cybernetics format, a new way of thinking was required.”
Some process steps proved to be particularly challenging, such as the introduction of biological material. This is because isolator technology requires gassing by hydrogen peroxide, which can be damaging to unprotected cell material.
Operating concept to be integrated into hospital processes
Now Optima and RBK are in the driver’s seat. The experts at Optima Pharma are working on developing functional modules, while the RBK is working on the operating concept and developing a concept for a clinical trial to demonstrate the suitability of the system for use in decentralized CAR-T cell production.
In Q2 2021, work began on building and testing the first functional modules. As a result, it is assured at an early stage that the living cells are not damaged, for example by excessive shear forces. At RBK, test operations will start in the summer of 2022. Even then, UKHD experts will still have an important role to play in making appropriate adjustments to any slight deviation. The ultimate goal is to produce a safe product that is given to the patient as an intravenous drip.
Possible use in hospitals and industry
If the ProCell for Patient model is a successful one, the intention is then to turn the prototype into a marketable product. Dr. Traube explains: “The system can be used in the future in both tumour treatment centres and in the pharmaceutical industry.” Because of its modular design the project partners are anticipating that the production platform can be used to produce different types of cell and gene therapeutics. These have great potential. By 2025, EU and US regulatory authorities anticipate ten to 20 new products per year.
In the long term, multiple hospitals and their patients may benefit from the ProCell for Patient project. First and foremost is the RBK. Prof. Aulitzky expects to obtain manufacturing approval three to six months after the project ends. The Universitätsklinikum Heidelberg, which will initially be supplied by the RBK, will also benefit from this. However, in the medium term, Prof. Schmitt wants to be one of the first to roll out the technology: “We want to set up a number of robot production isolators on a factory floor.” Other clinics are also interested. Consequently, Baden- Württemberg as a location will benefit by becoming a leader in technology for the decentralized production of personalized cell and gene therapeutics, and will help in gaining acceptance by healthcare providers and regulatory authorities. This means that in the future, patients will receive treatment more quickly, with the promise of successful treatment even when all other options have failed.