AI-based automated blood-drawing device looks set to make phlebotomy departments way more efficient
International Hospital speaks to Toon Overbeeke, CEO & co-founder, and Brian Joseph, commercial director & co-founder, of the start-up Vitestro about their automated blood drawing device for which they have recently received significant support from a Series A funding round.
International Hospital: Your start-up company, Vitestro, has developed an autonomous blood drawing device. We’ll come back to this device, but first, let’s look at the company. What inspired you to start Vitestro, and when did the idea originate?
The idea for Vitestro was conceived when Toon Overbeeke and Brian Joseph, entrepreneurs and business partners, discussed frustrations about the availability of care for their loved ones for many years. In Toon’s case, a friend’s father was very difficult to puncture due to chemotherapy. At the same time, Brian experienced long waiting times and logistical errors when one of his children needed regular blood check-ups.
These personal experiences showed the shortcomings of the most performed invasive procedure in the world: blood drawing. Together the founders saw an opportunity to improve patient care with autonomous phlebotomy.
When and where did you establish the company?
In Utrecht, the Netherlands, in 2017
Can you outline the initial processes/research that led to the company’s establishment?
We contacted many blood drawing departments in laboratories and hospitals to understand their problems. Next, we interviewed many patients and technical experts and got confirmation of their willingness to adopt autonomous blood drawing and its technical feasibility.
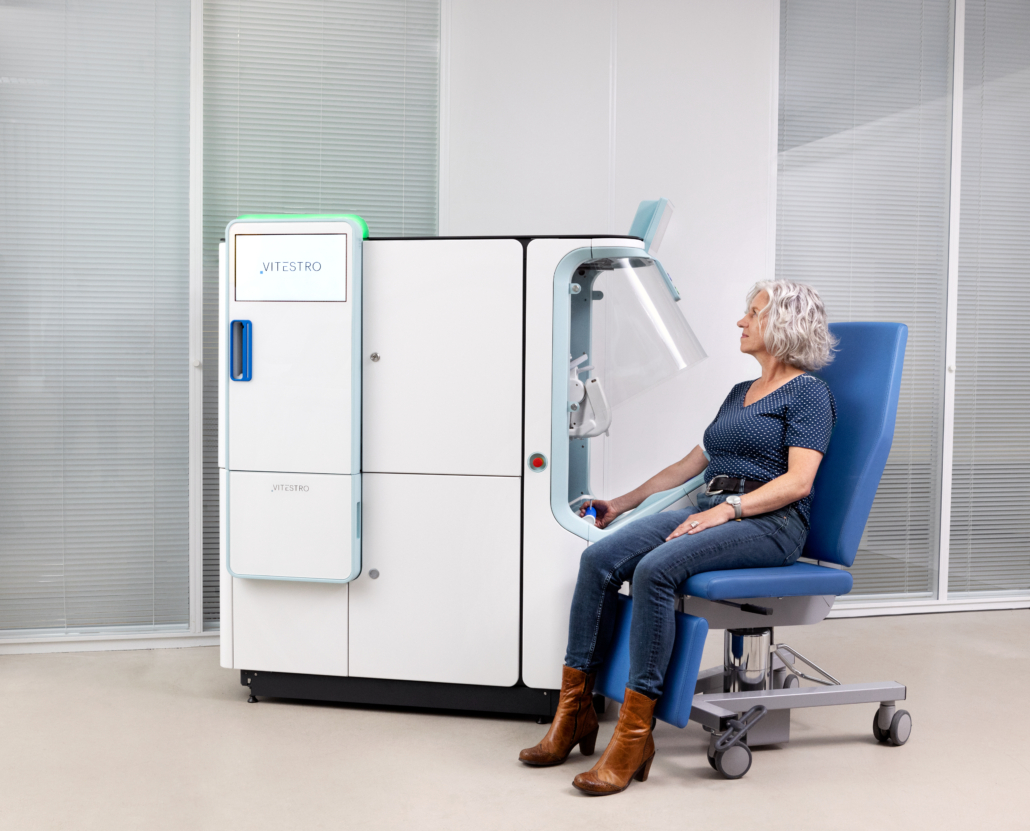
Can you explain how your autonomous blood drawing device works?
Vitestro’s device autonomously performs the whole venipuncture procedure carrying out all the activities usually done by a nurse: it applies a tourniquet, detects the vein, draws the blood, disposes of the needle, and places the bandage.
The ultrasound system gives an image of the cross-section of the patient arm in which the location of the vein can be determined. Subsequently, the device ensures the needle can be inserted into the vein with the required accuracy and safety.
What are the benefits of using an autonomous blood drawing device compared to traditional blood drawing methods?
The innovation will contribute to safe, consistent blood drawing, as well as help to solve the growing healthcare workforce shortage.
Vitestro’s vision is that one healthcare professional (nurse, phlebotomist or other) can supervise multiple devices simultaneously. The remaining staff can be reallocated to other parts of the hospital or laboratory. This provides major new possibilities in care delivery, increasing access to care by automating invasive patient procedures.
What were some of the challenges the Vitestro team faced during the development process – and how were they resolved?
The device consists of many subsystems, which need to be tested independently. To solve this challenge, we have designed the device in a modular way. In fact, we have divided the complete system into Line Replaceable Units. This will also ensure smooth maintenance when the device is in place with our customers.
Can you tell us about the clinical studies conducted on your device?
Vitestro conducts its clinical development activities together with leading hospitals and clinical laboratories in the Netherlands.
The study population includes a varied sample of volunteering patients. The results of these studies are used for technology development and optimization. From 2023 onwards, Vitestro will initiate studies for publication and regulatory approval.
How do you plan to ensure the safety and accuracy of your device during blood collection?
We have implemented many control measures to mitigate the risks as far as possible. We are very comfortable using our own device – which is important when bringing a medical device to the market. Accuracy is key for positioning the needle on sub-millimetre level in the vein – so this has been a focus of the team since the start of Vitestro.
You have recently received Series A funding. How will this funding be used?
Yes, we received €12 million from a Series A financing round. California-based Sonder Capital led the funding round alongside existing and new private investors with experience in the clinical laboratory and MedTech industry.
The proceeds will accelerate product development, prepare EU market authorization, and initiate production.
Can you discuss any potential partnerships or collaborations Vitestro is considering for the future?
We foresee potential partnerships or collaborations with diagnostic companies, laboratories, academic centres and suppliers.
What regulatory hurdles do you anticipate encountering during the market introduction of the device in Europe?
A prototype has been tested on more than 1,000 patients. Clinical studies with our final venipuncture device are in progress. After our pivotal studies, we will file for CE marking, which is required for market introduction in Europe.
When do you envisage the product coming to market – in Europe, the US and worldwide?
European market introduction is anticipated at the end of 2024. So that is our first focus.
Who is the target market?
We will initially focus on laboratories and hospitals that have larger phlebotomy departments.
Can you share any future product development or expansion plans beyond the autonomous blood drawing device?
There are many opportunities to use our technology other than for blood drawing, but first we will focus on bringing our device to the market.
How do you see the healthcare industry evolving with the integration of medical robotics technology like the Vitestro device?
Given the mounting shortages of healthcare personnel, there is a clear need for future-proof, autonomous systems to guarantee continuity of care. At the same time, medical robotics will make consistent and standardized outcomes available to everyone.
See:https://vitestro.com/
How can parties who are interested in investing in your company go about doing this?
We will raise a Series B in the next 24 months. Although there is no need for fundraising activities at the moment, we are always happy to be in touch with companies that can add value to ours.
Lastly, what are some of the key things you have learnt in this process that you can pass on to others who are about to begin this journey?
Hire a multidisciplinary team and establish the right team culture to develop something to benefit patients. And it is very important to have focus as a team!
Toon Overbeeke, CEO
& co-founder, Vitestro
Brian Joseph, commercial director
& co-founder, Vitestro
The first order
In April, this year, Amsterdam-based OLVG Lab, a leading Dutch clinical laboratory, announced they will become the first European healthcare provider to introduce multiple autonomous blood drawing devices manufactured by Vitestro. Both parties signed an agreement for the acquisition of the devices, which will be installed once CE marking has been obtained. OLVG Lab says the innovation will contribute to safe, consistent blood drawing, as well as helping to solve the growing healthcare workforce shortage.