Manipulating embryonic ‘biological clock’ could boost lab-grown blood stem cells
New research reveals how delaying inflammatory signalling can dramatically increase the number of blood stem cells formed during embryonic development, potentially aiding efforts to produce these cells in the laboratory.
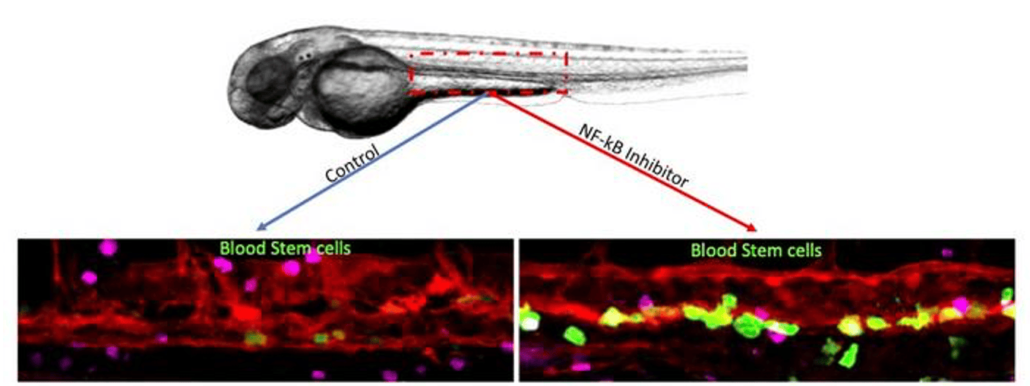
Magnified images show the increase in blood stem cell production that occurred when a second wave of inflammatory signaling was delayed in zebrafish embryos. The blood stem cells are colored green. © Clyde Campbell
Researchers at Iowa State University have made a significant discovery that could enhance the production of blood stem cells in laboratory settings. Their study, published in Nature Communications on 6 September 2024, sheds light on the intricate timing of inflammatory signals during embryonic development and how manipulating these signals can lead to a substantial increase in blood stem cell numbers.
The timing of inflammation
The research team, led by Raquel Espin Palazon and Clyde Campbell, assistant professors of genetics, development and cell biology at Iowa State University, built upon Espin Palazon’s previous work from a decade ago. That earlier research established the essential role of NF-kB, a protein network involved in triggering inflammation, in the formation of blood stem cells.
In their latest study, the scientists used zebrafish embryos to investigate the precise timing of NF-kB activation during blood stem cell development. They discovered that inflammatory signalling occurs in two distinct waves, acting as a biological clock to coordinate the conversion of vascular cells into blood stem cells.
“This was really a huge step forward for the lab-based production of blood stem cells. Those protocols are going to be more precise and efficient,” said Espin Palazon.
The importance of signal timing
The researchers found that the first wave of inflammatory signalling primes cells for transition, whilst the second wave prompts newly created stem cells to detach from blood vessels. Intriguingly, they observed that delaying the second wave of signalling led to a significant increase in blood stem cell proliferation.
Campbell described the moment of discovery: “I was the first person to see it through the microscope, and I nearly fell out of my chair. I yelled at Raquel, ‘What is this?’ It’s one of those moments you love to have in science. There are just a few times when you see something that blows you away. I expected to see maybe eight stem cells, and instead I saw hundreds.”
Implications for regenerative medicine
This finding has potential implications for regenerative medicine, particularly in the development of lab-grown, patient-derived blood stem cells. Such advancements could eventually replace the need for bone marrow transplants in treating blood disorders like leukaemia, lymphoma, and anaemia.
Current methods for culturing blood stem cells involve genetically reprogramming mature cells to behave like embryonic stem cells, then using these induced pluripotent stem cells to generate blood stem cells. However, these methods typically produce few functional cells.
“By manipulating that signalling, we can create a massive amount of blood stem cells,” Espin Palazon explained.
Translating findings to human cells
The Iowa State team collaborated with researchers at the Children’s Hospital of Philadelphia, who confirmed that NF-kB signalling exhibits similar timing patterns and effects in laboratory efforts to produce human blood stem cells.
To further this line of research, Espin Palazon and Campbell’s lab at Iowa State University will soon be equipped to study the process in-house. A £1.6 million, 5-year grant from the National Institutes of Health has funded both the current research and the creation of a cell culture lab capable of generating induced pluripotent stem cells.
Broader implications
The researchers anticipate that their methods and findings will have applications beyond blood stem cells, potentially informing studies on other types of stem cells, the ageing process, and patient-derived immunotherapy for cancer treatment.
“Inflammatory networks are required to start life, they keep us alive by fighting infections and viruses and, in the end, can cause our demise,” Campbell noted, highlighting the varied functions of inflammation signalling throughout life.
Future directions
As the field of regenerative medicine continues to advance, the insights gained from this study could prove crucial in optimising protocols for creating blood stem cells in laboratory settings. The ability to produce large numbers of functional blood stem cells could revolutionise treatments for a wide range of blood disorders, offering hope to patients who currently rely on bone marrow transplants.
With their new cell culture lab set to be operational by the end of the year, Espin Palazon and Campbell are poised to further explore the intricacies of stem cell development and the role of inflammatory signalling in this process.
As research in this area progresses, it may bring us closer to the goal of patient-specific, lab-grown blood stem cells for therapeutic use, potentially transforming the treatment landscape for blood disorders and beyond.
Reference:
Espin Palazon, R., Campbell, C., et al. (2024). p65 signaling dynamics drive the developmental progression of hematopoietic stem and progenitor cells through cell cycle regulation. Nature Communications, 15(1). https://doi.org/10.1038/s41467-024-51922-5

