Womed introduces first intrauterine adhesion barrier film to European market
Womed has launched its pioneering medical device designed to prevent intrauterine adhesions (IUA) across 14 European countries through new distribution partnerships with Kebomed Europe and Saesco Medical.
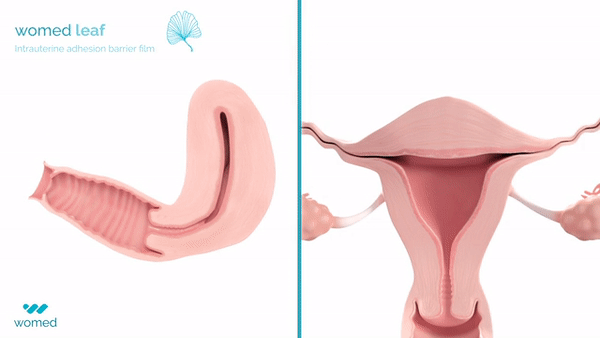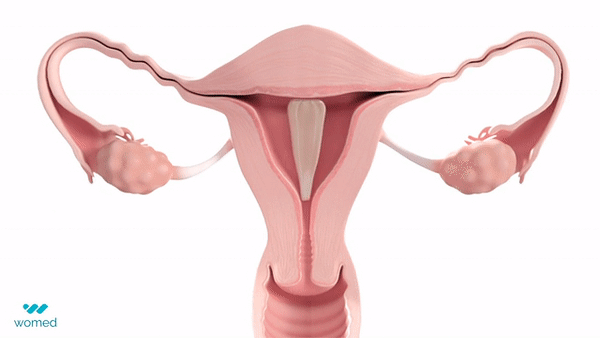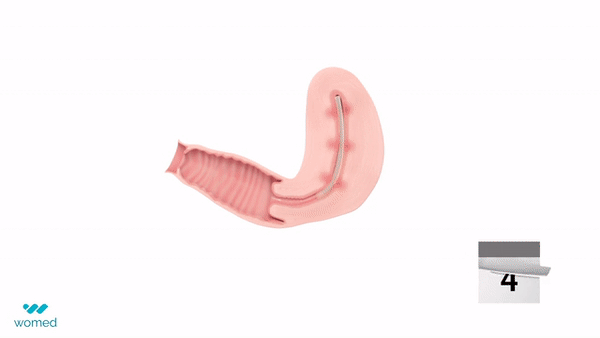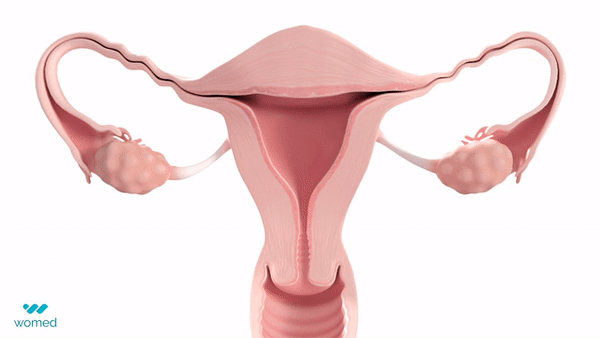
The device, Womed Leaf®, represents a significant advancement in uterine health management as the first physical barrier specifically developed to prevent adhesion formation following intrauterine procedures. The CE-marked product addresses a critical unmet need in fertility preservation for women undergoing procedures such as dilation and curettage or fibroid removal.
Clinical evidence demonstrates efficacy
Intrauterine adhesions, which occur in 20% to 45% of common gynaecological procedures, involve pathological binding of the uterine walls due to scarring. These adhesions frequently lead to infertility, recurrent miscarriages and chronic pelvic pain, with high recurrence rates following conventional treatment approaches.
The Womed Leaf device’s effectiveness was established through the PREG2 randomised clinical study involving 160 patients with moderate to severe IUA, positioning it as a potential new standard in IUA prevention and management.
Innovative design addresses structural challenges
“These partnerships mark the first step of a global launch that will make Womed Leaf the new gold standard in intrauterine adhesion prevention and treatment,” said Gonzague Issenmann, Co-founder and CEO of Womed.
The device features a soft, flexible film manufactured from the company’s proprietary polymer technology. Inserted similarly to an IUD following a uterine procedure, the film expands within the cavity to prevent contact between uterine walls for one week before being naturally and painlessly discharged.
European distribution network established
The distribution agreement divides European territories between two established medical device distributors with expertise in women’s health technologies.
“Womed Leaf addresses a significant unmet need in women’s health and we are excited to partner with Womed to bring it to our customers,” said Lars Melbye, Managing Director of Kebomed Europe, which will handle distribution across Northern and Central European markets including France, Germany and Scandinavia.
Saesco Medical, responsible for Southern European distribution, shares this enthusiasm. Jordi Fluvia, CEO of Saesco Medical, added: “We are committed to offering the latest advances in medical technologies, and Womed is bringing a true innovation in an indication that has been sorely lacking in meaningful improvements for the last two decades.”
- For more information, visit: womedtech.com