Wireless implant breakthrough offers personalised chronic pain relief
Researchers at the University of Southern California have developed a revolutionary wireless implant that could transform chronic pain management for millions of patients. The ultrasound-induced wireless implantable (UIWI) stimulator combines artificial intelligence with flexible bioelectronics to deliver personalised spinal cord stimulation without requiring batteries or invasive surgical procedures.
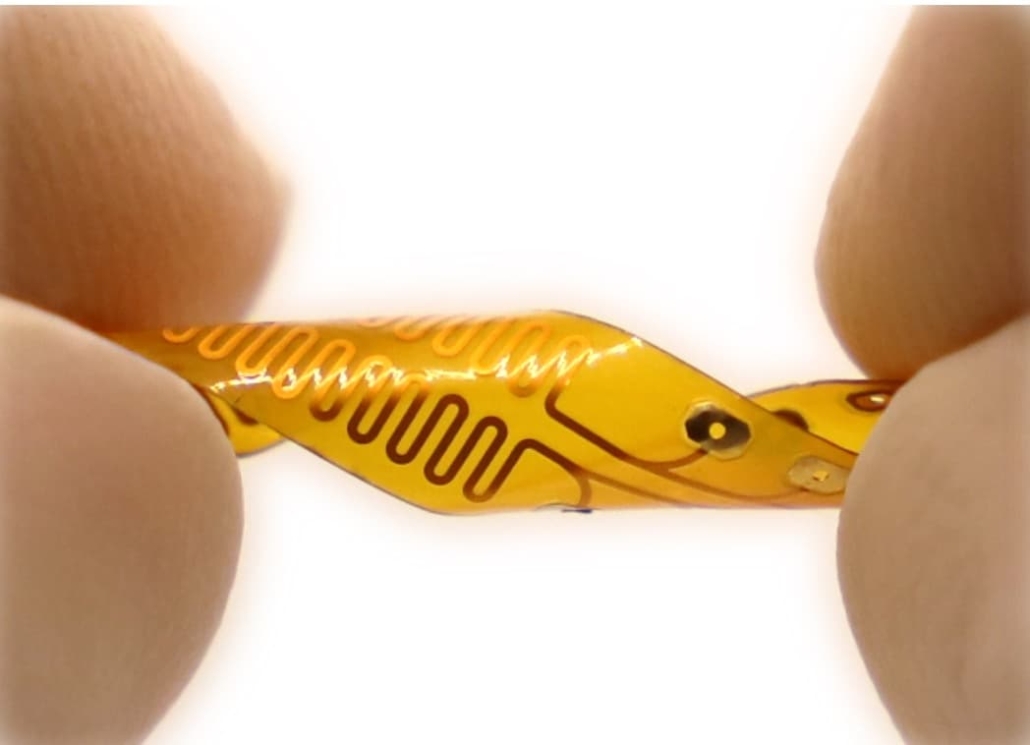
The new implantable device for chronic pain management is small and flexible. © The Zhou Lab at University of Southern California
The breakthrough technology, detailed in Nature Electronics on 12 May 2025, addresses the limitations of current pain management approaches that rely heavily on addictive opioid medications or bulky implantable devices requiring frequent battery replacements. With 51.6 million Americans living with chronic pain – 17 million experiencing high-impact symptoms that severely limit daily activities – this innovation represents a significant advancement in pain therapy.
Revolutionary wireless power delivery system
The UIWI stimulator eliminates the need for batteries through an innovative ultrasound-based power transmission system. The device receives energy from an external wearable ultrasound transmitter (WUT) that can be attached to the patient’s skin surface. This wireless approach removes the necessity for bulky implanted batteries and complex wired interfaces that often require repeated surgeries for replacement.
“What truly sets this device apart is its wireless, smart and self-adaptive capability for pain management,” said Professor Qifa Zhou, who led the research team. “We believe it offers great potential to replace pharmacological schemes and conventional electrical stimulation approaches, aligning with clinical needs for pain mitigation.”
The device converts mechanical ultrasound waves into electrical signals through the piezoelectric effect, utilising a miniaturised element made from lead zirconate titanate (PZT). The team optimised the design using a 1-3 composite structure that achieves superior electromechanical coupling compared to traditional bulk materials, resulting in enhanced acoustic-to-electric conversion efficiency.
AI-powered pain classification enables personalised treatment
A key innovation lies in the system’s ability to automatically classify pain levels and adjust treatment accordingly. The researchers developed a machine learning model based on the ResNet-18 neural network that analyses electroencephalogram (EEG) signals from the brain to categorise pain into three distinct levels: slight, moderate, and extreme pain.
The AI system demonstrated remarkable accuracy, correctly identifying pain states 94.8% of the time across 5,770 data samples. This classification enables the device to automatically adjust stimulation parameters, creating a closed-loop system that provides real-time, personalised pain management.
“By leveraging wireless ultrasonic energy transfer and closed-loop feedback system, this UIWI stimulator removes the necessity for bulky implanted batteries and allows for real-time, precisely adjustable pain modulation,” explained lead author Yushun Zeng.
Demonstrated efficacy in preclinical studies
The research team validated the technology through comprehensive testing in rodent models of chronic neuropathic pain. Animals with spared nerve injury received both mechanical stimuli (pin prick using Von Frey filaments) and thermal stimuli (infrared heat) to induce different pain levels.
Results demonstrated significant reductions in pain indicators across all stimulation categories. In behavioural studies, animals showed clear preferences for environments where the pain management system was activated, confirming the device’s therapeutic effectiveness.
The authors noted that “electrical stimulation set-ups for three classified pain models are demonstrated” with specific protocols: slight pain required 199 mV stimulation at 50 Hz, moderate pain needed 409 mV at 50 Hz, and extreme pain demanded 417 mV at 200 Hz.
Flexible design enables minimally invasive implantation
The UIWI stimulator features a flexible, bendable design that can conform to the spinal cord’s anatomy. Built on a flexible printed circuit board substrate, the device measures just 2×2×1 mm³ and incorporates six parallel electrode sets for enhanced stimulation coverage.
The lightweight bioelectronic design demonstrates “soft, flexible and three-dimensional twisting mechanical properties,” making it suitable for long-term implantation with minimal tissue disruption. The device is coated with biocompatible parylene film for electrical insulation and waterproofing, with only the stimulating electrodes exposed for neural contact.
Safety considerations and future development
Safety testing confirmed that the ultrasound transmission operates well within FDA-approved limits for acoustic intensity and mechanical index. Temperature monitoring showed increases of less than 0.5°C during operation, demonstrating thermal safety for extended use.
The authors acknowledge that “current technology for recording is implanting microelectrodes for invasive recordings, which involves health risks and mobility limitations.” Future development will focus on “accurate and low-risk wireless neural recording” to translate findings into clinical applications.
Looking ahead, the researchers envision significant miniaturisation possibilities. “Future designs could miniaturise the components further, enabling less invasive device implantation—for instance, with a syringe,” Zhou explained. The wearable ultrasound transmitter could evolve into an untethered, miniaturised device or even a wearable ultrasound array patch with combined imaging and energy delivery capabilities.
Clinical translation pathway
The technology represents a significant step towards addressing the opioid crisis by providing non-addictive pain management alternatives. The authors conclude that “these findings highlight the potential of ultrasonic implantable electronics in clinical and translational chronic pain management.”
Future iterations could be controlled by smartphone software, offering robust personalised pain management that adapts to individual patient needs and pain fluctuations over time.
Reference
Zeng, Y., Gong, C., Lu, G., Wu, J., Wan, X., Yang, Y., … & Zhou, Q. (2025). A programmable and self-adaptive ultrasonic wireless implant for personalized chronic pain management. Nature Electronics, 8, 437-449. https://doi.org/10.1038/s41928-025-01374-6

