Smart bandage technology advances to human trials with promising results
Caltech researchers have successfully implemented their innovative iCares smart bandage system in human patients with chronic wounds, demonstrating real-time biomarker detection capabilities that could transform wound care management.
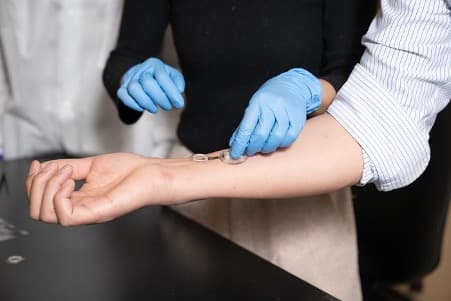
The iCares smart bandage on Wei Gao’s arm. The bandage is composed of a flexible, biocompatible polymer strip that can be 3D printed at low cost. © Lance Hayashida/Caltech
Chronic wounds represent a significant healthcare challenge worldwide, often requiring intensive monitoring and treatment that strains medical resources and diminishes patient quality of life. A promising solution has emerged from California Institute of Technology (Caltech) researchers, who have recently demonstrated successful human implementation of their advanced smart bandage technology.
Microfluidic innovation enables continuous wound monitoring
The iCares smart bandage, developed by Professor Wei Gao’s team at Caltech, has successfully progressed from animal testing to human trials. In a study published on 23 April 2025 in Science Translational Medicine, researchers demonstrated the bandage’s ability to continually sample wound fluid from 20 patients suffering from chronic wounds related to diabetes or poor circulation.
“Our innovative microfluidics remove moisture from the wound, which helps with healing. They also make sure that samples analysed by the bandage are fresh, not a mixture of old and new fluid. To get accurate measurements, we need to sample only the newest fluid at a wound site,” explains Professor Gao, who also serves as a Heritage Medical Research Institute Investigator. “In this way, iCares can watch in real time for important biomarkers of inflammation and infection.”
The technology represents a significant advancement over traditional wound monitoring methods, which typically rely on visual assessment by healthcare professionals during scheduled appointments.
Advanced engineering enables early detection capabilities
The iCares system integrates three specialised microfluidic components: a membrane that extracts wound fluid, a bioinspired component that transports the fluid to sensors, and a micropillar module that removes sampled fluid from the bandage.
This sophisticated fluid management system enables the detection of crucial biomarkers such as nitric oxide (indicating inflammation) and hydrogen peroxide (signalling infection) up to three days before patients experience symptoms. The early detection window could prove invaluable for timely intervention and improved patient outcomes.
The bandage itself comprises a flexible, biocompatible polymer strip manufactured via cost-effective 3D printing techniques. It features a nanoengineered disposable sensor array integrated with a reusable printed circuit board that processes signals and transmits data wirelessly to a smartphone or other user interface.
Machine learning enhances diagnostic capabilities
Beyond physical engineering innovations, the research team has developed a machine learning algorithm that analyses multiparameter sensor data to classify wounds and predict healing timeframes. According to the study, this algorithmic approach demonstrates accuracy comparable to expert clinical assessment.
This integration of artificial intelligence with physical sensing technology represents a promising direction for objective wound evaluation, potentially reducing reliance on subjective visual assessments and enabling more precise treatment protocols.
From laboratory to clinical application
The iCares technology has undergone rigorous testing, initially in diabetic mice and subsequently in 20 human patients with chronic wounds, plus two additional patients monitored before and after surgery.
The bandage continuously monitors multiple parameters including reactive species such as nitric oxide, hydrogen peroxide, and oxygen, along with pH and temperature. This comprehensive data collection enables a more nuanced understanding of wound status than traditional methods can provide.
For patients with chronic wounds – particularly those resulting from conditions like diabetes that impair natural healing processes – this technology offers hope for more effective management and potentially improved healing outcomes.
The research was supported by grants from several organisations including the National Institutes of Health, National Science Foundation, American Cancer Society, Army Research Office, US Army Medical Research Acquisition Activity, and the Heritage Medical Research Institute, with additional infrastructure support from Caltech’s Kavli Nanoscience Institute.
Reference
Wang, C., Fan, K., Lasalde-Ramirez, J. A., et. al. (2025). A microfluidic wearable device for wound exudate management and analysis in human chronic wounds. Science Translational Medicine. https://doi.org/10.1126/scitranslmed.adt0882

