Scientists develop portable bone printer to create custom implants during surgery
Scientists have engineered a portable 3D printing device that produces bone grafts directly onto fractures during surgery. The technology enables real-time fabrication of customised implants without pre-operative preparation, whilst incorporating antibiotics to prevent infection and promoting natural bone regeneration at the grafting site.
© Device / Jeon et al.
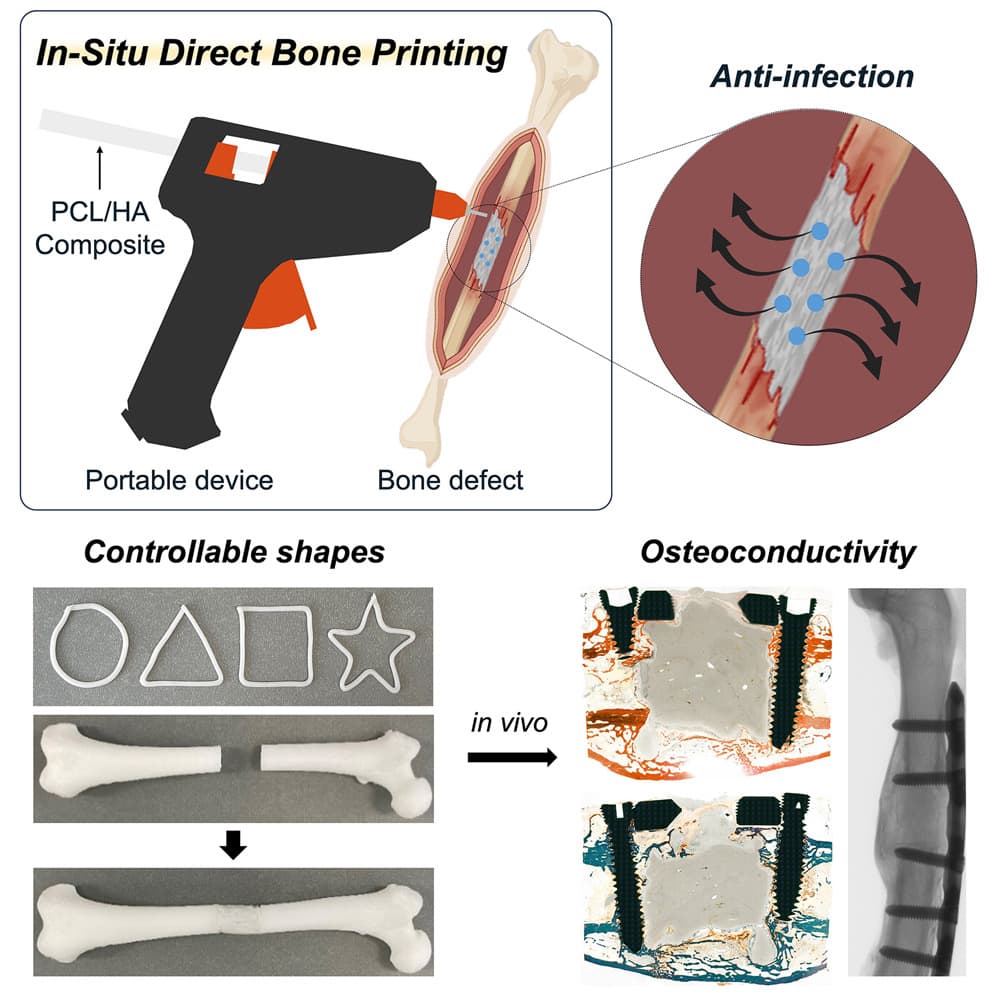
Scientists have developed a tool made from a modified glue gun that can 3D print bone grafts directly onto fractures and defects during surgery. The tool, described 5 September in the Cell Press journal Device, allows scientists to quickly create complex bone implants without the need for prefabricating in advance.
Traditional bone implants utilise metal, donor bone, or pre-manufactured 3D-printed materials that require extensive pre-operative planning for irregular bone fractures. “Our proposed technology offers a distinct approach by developing an in situ printing system that enables a real-time fabrication and application of a scaffold directly at the surgical site,” says Jung Seung Lee, co-author and associate professor of biomedical engineering at Sungkyunkwan University, Seoul, South Korea. “This allows for highly accurate anatomical matching even in irregular or complex defects without the need for preoperative preparation such as imaging, modelling, and trimming processes.”
Novel composite material enables precision printing
The device utilises a specialized filament comprising hydroxyapatite (HA) and a biocompatible thermoplastic called polycaprolactone (PCL). PCL can liquify in temperatures as low as 60°C, which when applied with a heat-modified glue gun, is cool enough to prevent tissue damage during surgical application while being able to conform to the jagged grooves of fractured bone.
“By adjusting HA content and the molecular weight of PCL, we optimized the mechanical and biological properties of the scaffolds for structural stability, long-term performance, and osteoconductivity,” the authors explain.
The researchers demonstrated precise control over implant properties by varying the hydroxyapatite-to-PCL ratio. Higher molecular weight PCL formulations with 25% hydroxyapatite content exhibited optimal mechanical properties, achieving compressive strengths exceeding 900 Newtons whilst maintaining biocompatibility.
Integrated antibiotic delivery reduces infection risk
Post-surgical infection remains a significant concern in orthopaedic procedures, affecting up to 33% of patients with open fractures. The research team addressed this challenge by incorporating vancomycin and gentamicin antibiotics directly into the printing filament.
In both petri dish culture and liquid medium, the filament scaffold successfully inhibited the growth of E. coli and S. aureas, two common bacteria prone to cause infection post-surgery. Due to physical properties of HA and PCL within the filament, the drugs are released slowly and are able to diffuse directly onto the surgical site over several weeks.
“This localized delivery approach offers meaningful clinical advantages over systemic antibiotic administration by potentially reducing side effects and limiting the development of antibiotic resistance, while still effectively protecting against postoperative infection,” says Lee.
The controlled release mechanism enables sustained antimicrobial activity whilst minimising systemic exposure, addressing both therapeutic efficacy and antibiotic stewardship concerns.
Surgical efficiency and real-time customisation
The portable nature of the device enables surgeons to modify implant geometry during procedures. “Because the device is compact and manually operated, the surgeon can adjust the printing direction, angle, and depth during the procedure in real time,” says Lee. “Also, we demonstrated that this process could be completed in a matter of minutes. This highlights a significant advantage in terms of reducing operative time and improving procedural efficiency under real surgical conditions.”
The system’s temperature control mechanism prevents thermal tissue damage through modified heating elements that operate at 80°C rather than conventional glue gun temperatures exceeding 100°C. Surface temperature measurements confirmed that extruded material reaches body temperature within 40 seconds, ensuring biocompatibility.
Superior bone regeneration demonstrated in animal trials
As a proof of concept, the device was tested on severe femoral bone fractures in rabbits. Within 12 weeks after surgery, the team found no signs of infection or necrosis and greater bone regeneration outcomes when compared to rabbits grafted with bone cement – a sealing compound commonly used for treating bone defects.
Micro-computed tomography analysis revealed significant improvements in bone regeneration parameters. The PCL/HA scaffolds demonstrated enhanced bone volume, surface area, and mechanical stability compared to conventional bone cement treatments.
“The scaffold was designed not only to integrate biologically with surrounding bone tissue but also to gradually degrade over time and be replaced by newly formed bone,” says Lee. “The results showed that the printing group exhibited superior outcomes in key structural parameters such as bone surface area, cortical thickness, and polar moment of inertia, suggesting more effective bone healing and integration.”
Clinical translation pathway
The research team acknowledges several development requirements before clinical implementation. “Clinical adoption will require standardised manufacturing processes, validated sterilisation protocols, and preclinical studies in large animal models to meet regulatory approval standards,” says Lee. “If these steps are successfully achieved, we vision this approach becoming a practical and immediate solution for bone repair directly in the operating room.”
Future research directions include optimising antibacterial efficacy, enhancing mechanical properties through polymer modifications, and developing temperature control systems for irregular geometries. The team also plans to investigate porogen incorporation to improve nutrient exchange within scaffolds.
The in situ printing device enables precise and efficient scaffold fabrication directly onto the defect site, offering a versatile platform with various thermoplastics for treating different types of bone defects, the authors conclude in their paper.
This technology represents a paradigm shift from pre-manufactured implants towards patient-specific, real-time bone reconstruction that could significantly improve surgical outcomes whilst reducing procedural complexity and cost.
Reference
Jeon, I. Y., Jeon, Y. M., Choi, J. H., et. al. (2025). In situ printing of biodegradable implant for healing critical-sized bone defect. Device, 3, 100873. https://doi.org/10.1016/j.device.2025.100873

