Philips’ Duo Venous Stent System receives FDA approval and completes first implant
Royal Philips has announced the successful first implantation of its Duo Venous Stent System following premarket approval from the U.S. Food and Drug Administration. The device is indicated for treating symptomatic venous outflow obstruction in patients with chronic venous insufficiency (CVI).
First implantation marks milestone in CVI treatment
On 11 June, Dr Erin Murphy, vascular surgeon and director of the Venous and Lymphatic Program at the Sanger Heart & Vascular Institute, Atrium Health, in Charlotte, N.C., performed the first implantation of Philips’ Duo Venous Stent System outside of a clinical trial. Dr Murphy was an investigator in the VIVID study, which contributed to the device’s FDA approval.
Deep venous disease, affecting 25 million people globally, stems from venous thromboembolism – the third most common cardiovascular disease. The complexities of deep venous anatomy and obstructions present significant mechanical challenges for treatment.
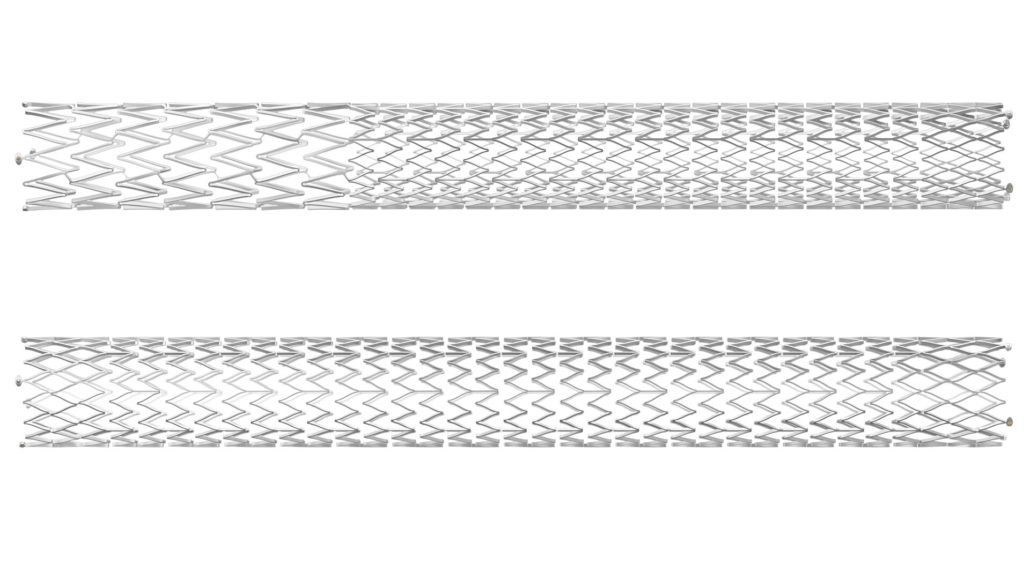
Innovative design addresses venous anatomy challenges
The Duo Venous Stent System comprises two stents – Duo Hybrid and Duo Extend – available in various sizes. The Duo Hybrid features an integrated design with multiple zones of differing mechanical properties within a single stent. For longer lesions, the Duo Extend can be smoothly overlapped with the Duo Hybrid to extend therapy.
Dr Kush Desai, an interventional radiologist and associate professor at Northwestern University in Chicago, who was a leading enroller and investigator for the VIVID study, commented on the device’s unique design: “Duo is the first stent that offers a differential design for the challenges of venous anatomy – a focal area that withstands the forces of compression as well as the flexibility to accommodate curvature of the vessel.”
This design aims to minimise the risk of stent fracture and corrosion while providing an option for stenting within caudal veins with smaller diameters.
VIVID study demonstrates safety and efficacy
The VIVID study, a global, prospective, multi-centre, single-arm, non-blinded clinical trial, evaluated the safety and efficacy of the Philips Duo Venous Stent System in treating nonmalignant iliofemoral occlusive disease. The study enrolled 162 subjects at 30 centres, including patients with non-thrombotic iliac vein lesion (NIVL), post-thrombotic syndrome (PTS), and acute deep vein thrombosis (aDVT).
The study met all primary safety and efficacy performance goals:
- The 12-month effectiveness endpoint for primary patency reached 90.2%, exceeding the performance target goal of 77.3%.
- The 12-month primary safety endpoint of 98.7% surpassed the corresponding performance goal of 89%.
Additionally, quality of life and venous functional assessments – including Clinical-Etiology-Anatomy-Pathophysiology (CEAP), Venous Clinical Severity Score (VCSS), Villalta, EQ-5D-3L, and VEINES scores – showed sustained improvements compared to baseline at 12 months.
Dr Mahmood Razavi, principal investigator and interventional radiologist with St. Joseph Vascular in Orange County, California, commented: “The VIVID study’s 12-month results demonstrate the safety and efficacy of the Duo Venous Stent System in the treatment of chronic venous insufficiency, a vascular condition affecting millions of people worldwide. Duo represents a meaningful addition to the tools that clinicians can use to treat CVI patients, especially when used in conjunction with intravascular ultrasound, or IVUS. Ultimately, the new device promises to enable excellent clinical outcomes and drive significant quality of life improvements.”
Intravascular ultrasound: A key component in venous stenting
The VIVID study was the first clinical trial to mandate IVUS use for lesion assessment and stent sizing prior to device implantation. Previous research has shown that IVUS supports accurate diagnosis of venous disease and has been demonstrated to change 57% of treatment plans compared to venography alone. Philips reports that intravascular imaging is now used in more than 70% of venous stent procedures.
Heather Hudnut Page, Vice President and Business Leader of Peripheral Vascular at Philips, emphasised the company’s commitment to innovation in interventional procedures: “The launch of the Duo Venous Stent System represents another step forward in achieving our aspiration to innovate interventional procedures with advanced medical technology. In this context, we look forward to bringing the combined offering of intravascular ultrasound and Duo to the interdisciplinary teams – from vascular surgeons to interventional radiologists and interventional cardiologists – who share our overarching goal of enhancing patient care.”
The Duo Venous Stent System, developed by Vesper Medical, Inc., a wholly owned subsidiary of Philips, is being marketed under the Philips brand. As with all medical devices, it is subject to regulatory restrictions, with federal law in the United States limiting its sale to licensed healthcare practitioners or by their order.
This advancement in venous stenting technology, coupled with the widespread adoption of intravascular ultrasound, represents a significant step forward in the treatment of chronic venous insufficiency. The combined approach offers clinicians enhanced diagnostic accuracy and treatment options, potentially leading to improved outcomes for patients suffering from this common vascular condition.
- For more information, visit: Philips.com