Paediatric study for Bayer’s investigational MRI contrast agent gadoquatrane in children meets primary and secondary endpoints
Bayer has revealed results from the QUANTI Pediatric study, adding to the safety and efficacy data of the investigational low-dose MRI gadolinium-based contrast agent (GBCA) gadoquatrane.
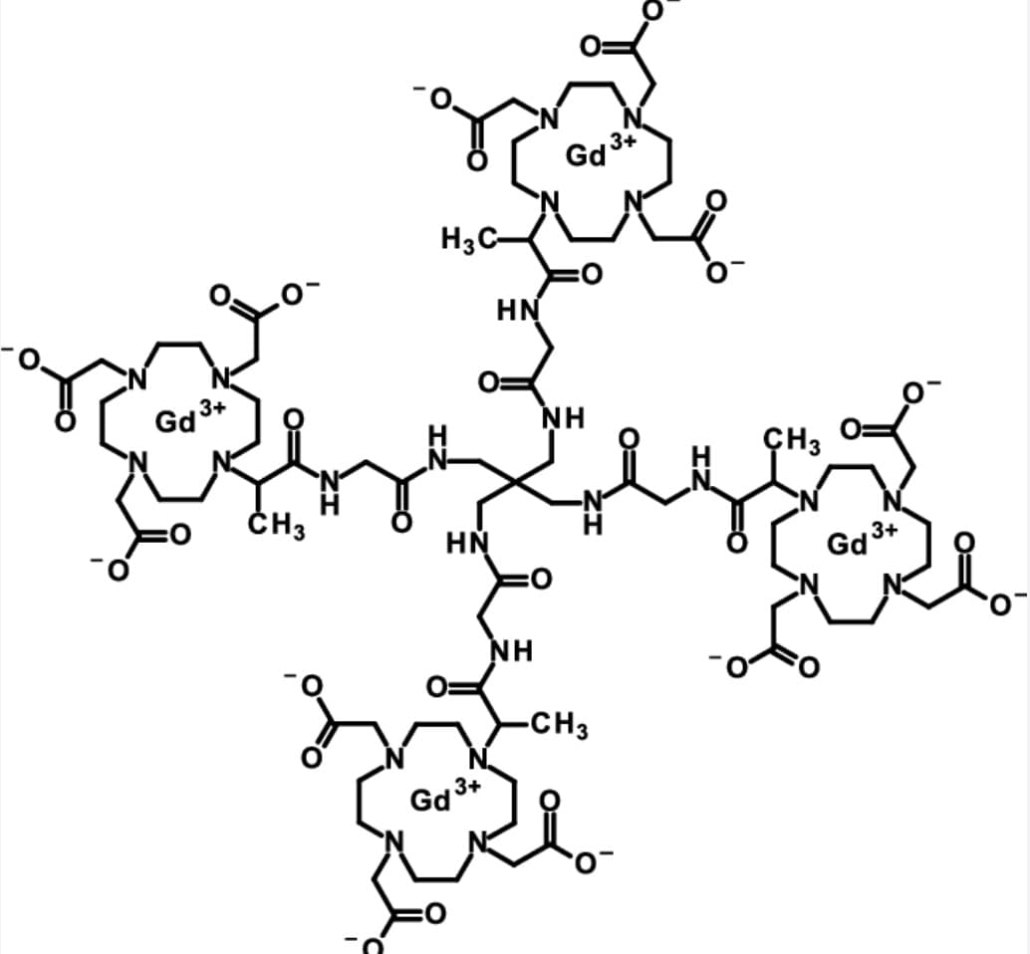
gadoquatrane
QUANTI Pediatric evaluated gadoquatrane in children with known or suspected disease undergoing contrast-enhanced magnetic resonance imaging (MRI) at a gadolinium dose of 0.04 mmol Gd/kg body weight, representing a dose reduction of 60 percent compared to the macrocyclic GBCAs dosed at 0.1 mmol Gd/kg body weight. The study met the primary and secondary endpoints, assessing the pharmacokinetic and safety profile of gadoquatrane. Similar pharmacokinetic behaviour was observed in the paediatric population, indicating that diagnostic performance of gadoquatrane in adults can also be applied to children. Details of QUANTI Pediatric were presented as late-breaking clinical trial data at this year’s annual congress of the Radiological Society of North America (RSNA) taking place in Chicago, USA, from November 30 to December 4, 2025.
QUANTI Pediatric is part of Bayer’s pivotal QUANTI clinical development program, which also encompassed two multinational Phase III studies in adults. Based on positive QUANTI data, including the paediatric study, Bayer has submitted applications for marketing authorization of gadoquatrane in markets around the world, such as Japan, the U.S., the EU, and China, with further markets to follow in the next months. If approved, gadoquatrane would become the lowest dose macrocyclic gadolinium-based contrast agent available in the respective markets.
Contrast-enhanced MRI
“Contrast-enhanced MRI is increasingly used to assist in diagnosis and monitoring of certain conditions, from newborns to adulthood,” said Talissa Altes, MD, Professor and Chair of Radiology at the University of Missouri, USA. “MRI is particularly valuable in paediatric care due to its non-invasive nature: it supports for example the diagnosis and follow-up of potential tumours as well as various neurological diseases such as multiple sclerosis in children. As MRI contrast agents commonly contain gadolinium, it can be especially relevant for patient groups that require multiple MRI exams over their lifetime, like paediatric patients, to have a low-dose contrast agent option to reduce lifetime exposure.”
An estimated 1.5 million contrast-enhanced MRIs are performed in children annually around the world – and this number is rising by 5% per year.
“Contrast-enhanced MRI serves as a crucial tool for disease detection and the ongoing condition management, including in children, and Bayer is committed to driving innovation in this important area,” said Dr. Konstanze Diefenbach, Head of Radiology Research & Development at Bayer’s Pharmaceuticals Division. “The QUANTI Pediatric results highlight gadoquatrane’s potential as a low-dose MRI contrast agent for children. A low dose is also in line with health authorities and scientific bodies which advise using the lowest dose required to obtain the needed clinical information. We look forward to ongoing collaborations with regulatory authorities around the world to make gadoquatrane accessible to patients and their healthcare providers as swiftly as possible.”
About QUANTI Pediatric and the Phase III development program QUANTI
QUANTI Pediatric was a multicentre, prospective, open-label study to evaluate the pharmacokinetics, safety and tolerability of gadoquatrane in children from birth to under 18 years old. The observed pharmacokinetic behaviour of gadoquatrane in children was similar to that in adults. The safety profile was in line with previous findings on gadoquatrane and other macrocyclic GBCAs. Additionally, the study assessed diagnostic performance in the paediatric population. Results show that gadoquatrane improved visualization and other key parameters when compared to pre-contrast MRI scans.
The pivotal QUANTI clinical development program for gadoquatrane consisted of two large multinational, randomized, prospective double-blind, crossover Phase III studies – QUANTI CNS (Central Nervous System) and QUANTI OBR (Other Body Regions) – as well as the QUANTI Pediatric study. In total, 808 patients – including 93 children – in 15 countries participated in the program. The results of the QUANTI studies show that gadoquatrane met the primary and secondary efficacy endpoints of the studies assessing visualization parameters and lesion detection, while reducing the gadolinium dose by 60 percent compared to the macrocyclic GBCAs dosed at 0.1 mmol Gd/kg body weight. In all studies, the observed safety profile was generally consistent with previous data on gadoquatrane and other macrocyclic GBCAs. No new safety signals were observed.
Further study data are planned to be presented at upcoming scientific meetings.
About gadoquatrane
Gadoquatrane is Bayer’s investigational extracellular macrocyclic contrast agent for contrast enhancement in MRI. This low-dose gadolinium-based contrast agent features a distinct tetrameric structure with high stability and high relaxivity.
About Radiology at Bayer
Building on a century of expertise, Bayer is committed to innovative products and high- quality services in diagnostic imaging to enhance patient care. Its leading radiology portfolio features contrast agents and devices for precise administration across modalities including computed tomography (CT), X-ray and magnetic resonance imaging (MRI), and positron emission tomography (PET). Bayer’s comprehensive offerings also include informatics solutions. In 2024, Bayer’s radiology products generated €2.1 billion in sales. Bayer continues to advance research and innovation in medical imaging, including the integration of AI.
For more information, visit: www.bayer.com

