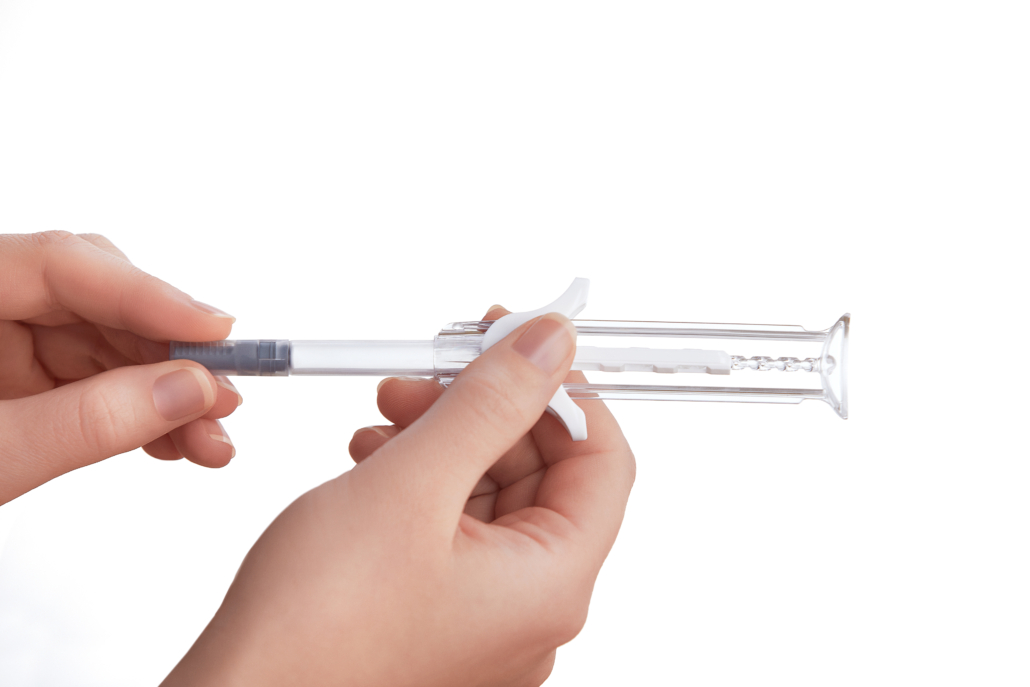
Owen Mumford’s novel UniSafe 1mL safety device for pre-filled syringes approved as a combination product in Asia
Owen Mumford Pharmaceutical Services’ UniSafe 1mL safety device for pre-filled syringes has been approved as a combination product in Asia. In Europe regulatory approval has also been granted for UniSafe 1mL and the product is now available as a combination product with a drug for treatment of rheumatoid arthritis.
UniSafe 1mL is a safety device for prefilled syringes that provides a variety of benefits to both pharmaceutical companies and their patients due to the unique, spring-free, patented design. Engineered without a spring to reduce complexity and cost, as well as preventing the risk of pre-activation in transit, during manufacturing or the removal from packaging, UniSafe 1mL is intuitive to patients and is used in the same way as a conventional pre-filled syringe with no additional steps. In addition, the plunger rod has an integrated design so that it cannot be pulled out when removing the needle shield or the device from its packaging, eliminating the risk of accidental drug spillage and compromised sterility.
The innovative design of UniSafe allows it to be simply tailored to different fill volumes, providing flexibility for those pharmaceutical companies who have drug candidates with multiple doses required for subcutaneous injection.
Further to this announcement and as part of the UniSafe platform, UniSafe 2.25 is now also fully industrialised and available. UniSafe 2.25 is designed to meet the needs of the growing biologic and biosimilar market, where subcutaneous injections of volumes greater than 1mL are often required. It is compatible with standard 2.25mL prefilled syringes with both cropped and small round flanges. UniSafe 2.25 shares many of the same design features as the 1mL and both products are intuitive to use with the same injection technique as a pre-filled syringe.
“The launch of our unique, patented springless design for the UniSafe platform is another milestone in our patient-centric innovation strategy,” said Michael Earl, Director, Owen Mumford Pharmaceutical Services. “Not only does our device look less intimidating for users, but the absence of a spring means that patients have complete visibility to check for drug clarity prior to injecting, while also ensuring the full dose has been delivered. There is also no risk of accidental pre-activation, giving our customers peace of mind that there will be no drug leakage or waste at any stage from dispatch to administration of the medication. As part of our scale up strategy for UniSafe we have already made significant investments in multi-cavity tooling and fully automated assembly to be able to deal with the anticipated demand for this platform.”
Both UniSafe 1mL and 2.25 have the option to substitute coloured finger flanges to provide branding identity or help distinguish between different medication dosages. In addition, UniSafe 1mL has the choice of using extended finger flanges to help those patients with dexterity or strength challenges.