Novel water-powered dressing accelerates wound healing through electrical stimulation
Researchers have developed an innovative, low-cost wound dressing that harnesses water-activated electrical stimulation to significantly accelerate healing in diabetic mice, potentially offering a game-changing solution for chronic wound treatment.
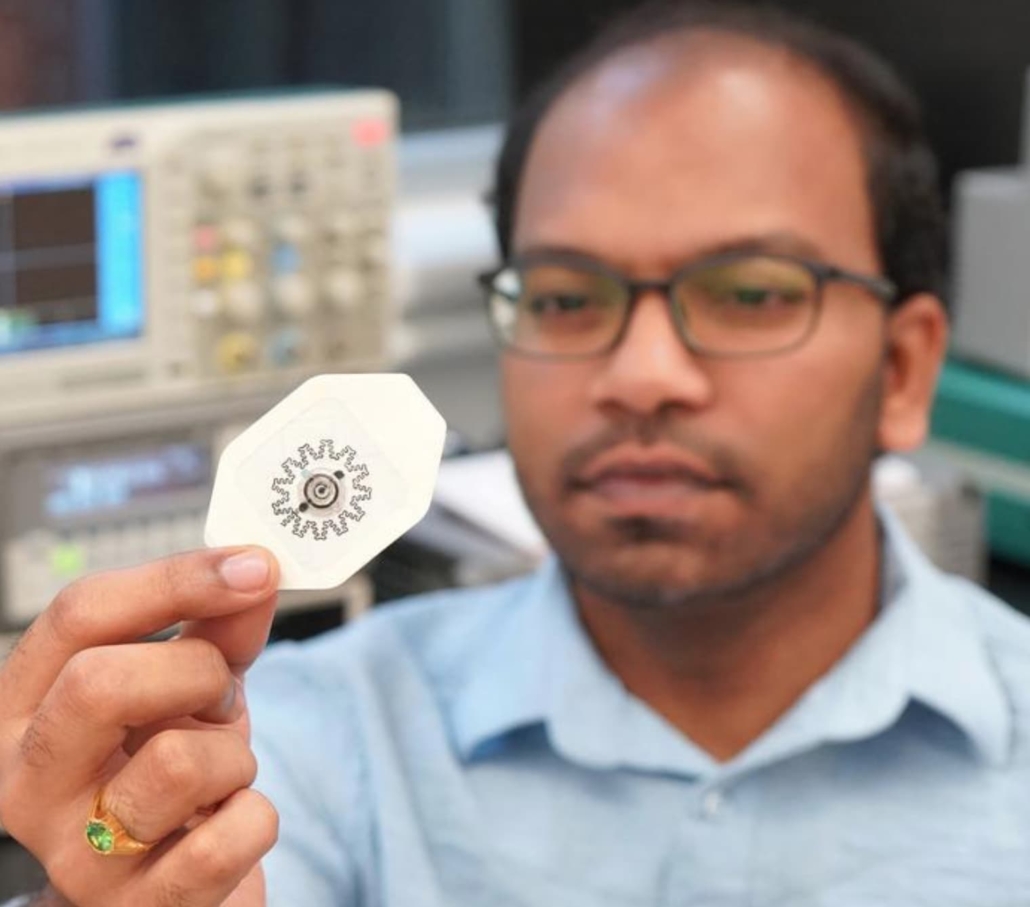
Researcher Rajaram Kaveti holds the water-powered, electronics-free dressing (WPED) for electrical stimulation of wounds. © Gurudatt Nanjanagudu Ganesh
A groundbreaking study published in Science Advances [1] describes a new approach to wound healing that could revolutionise the treatment of chronic wounds, particularly in diabetic patients. Researchers from Northwestern University have developed a water-powered, electronics-free dressing (WPED) that delivers electrical stimulation to wounds, promoting faster closure and improved healing outcomes. This novel technology, which is both cost-effective and easy to use, has shown promising results in preclinical trials, matching or surpassing the efficacy of more expensive treatments.
The chronic wound challenge
Chronic wounds affect approximately 2% of the US population and pose significant risks of amputation and mortality. Current treatments for these persistent wounds are often expensive, complex, and only moderately effective. While electrotherapy has shown promise as a cost-effective treatment, its reliance on bulky equipment has limited its clinical use.
The researchers sought to address this limitation by developing a self-contained, flexible dressing that can deliver electrical stimulation without the need for external power sources or complex electronics.
“Our goal here was to develop a far less expensive technology that accelerates healing in patients with chronic wounds,” said Amay Bandodkar, co-corresponding author of the work and an assistant professor of electrical and computer engineering at North Carolina State University. “We also wanted to make sure that the technology is easy enough for people to use at home, rather than something that patients can only receive in clinical settings.”
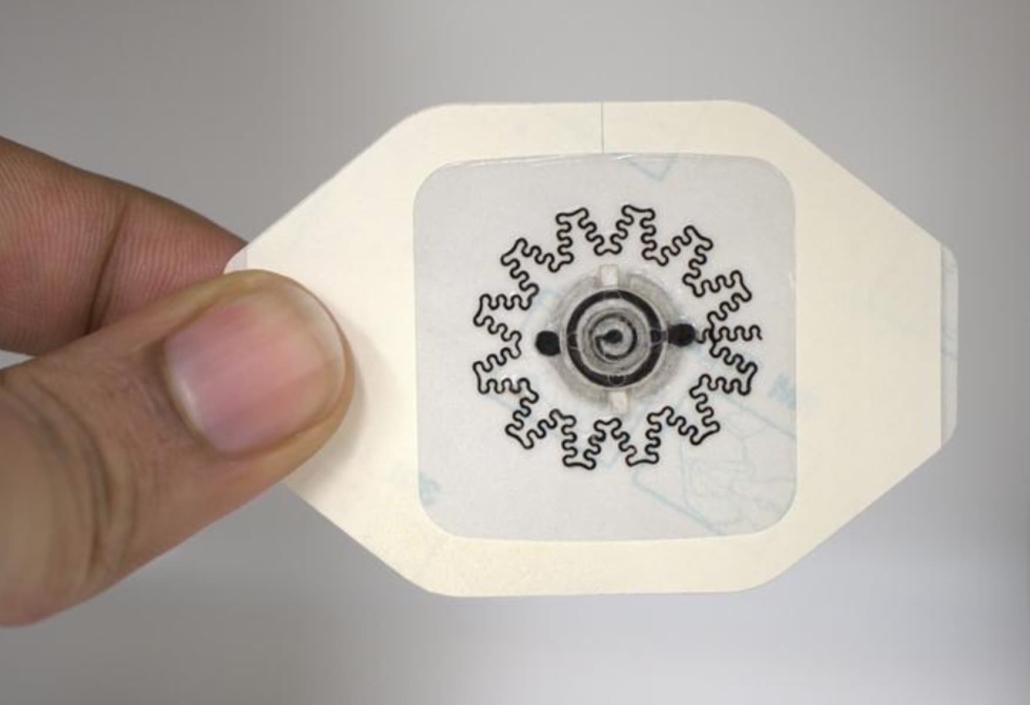
The water-powered, electronics-free dressing (WPED) for electrical stimulation of wounds. © Rajaram Kaveti
How the dressing works
The WPED consists of a biocompatible magnesium-silver/silver chloride battery and a pair of stimulation electrodes integrated into a commercial bandage. When water is added to the dressing, it activates the battery, creating a radial electric field that stimulates the wound.
The design of the dressing is ingeniously simple. It includes a ring-shaped battery with a dry cellulose separator impregnated with sodium chloride. The battery remains inactive until water is introduced through an inlet pad. A ‘check’ pad visually indicates when the separator is fully hydrated and the battery is activated.
Once activated, the battery produces a voltage of approximately 1.5V across the stimulation electrodes. The central disk electrode is connected to the magnesium anode (negative), while the outer ring electrode is attached to the silver/silver chloride cathode (positive). This configuration creates a radial electrical field pointing towards the wound centre, mimicking the natural endogenous electrical field crucial for promoting healing.
“That electric field is critical, because it’s well established that electric fields accelerate healing in chronic wounds,” said Rajaram Kaveti, co-first author of the study and a post-doctoral researcher at NC State.
The electrodes are designed in a way that allows them to bend with the bandage and conform to the surface of the chronic wounds, which are often deep and irregularly shaped.
“This ability to conform is critical, because we want the electric field to be directed from the periphery of the wound toward the wound’s centre,” said Kaveti. “In order to focus the electric field effectively, you want electrodes to be in contact with the patient at both the periphery and centre of the wound itself. And since these wounds can be asymmetrical and deep, you need to have electrodes that can conform to a wide variety of surface features.”
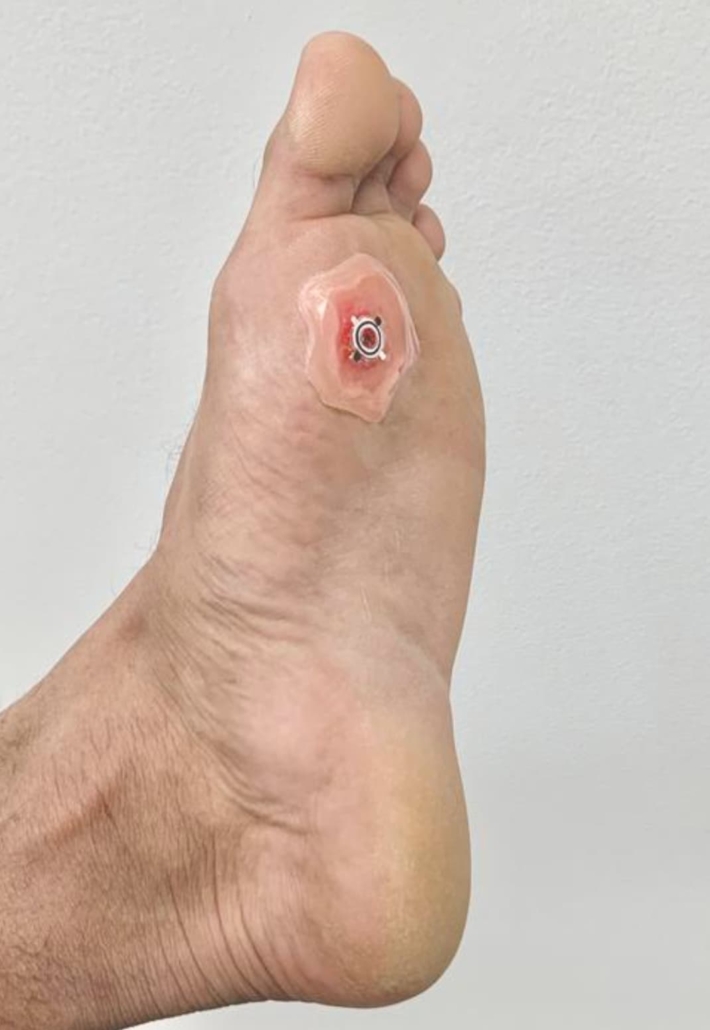
WPED applied to a dummy wound on a human foot. © Rajaram Kaveti
Impressive preclinical results
The researchers tested the WPED in a diabetic mouse model of impaired wound healing. The results were striking: by day 11 post-injury, 75% of wounds treated with the active WPED had fully closed, compared to only 12.5% in the sham device group and 0% in the control group treated with standard occlusive dressings.
Further analysis revealed that WPED-treated wounds exhibited several indicators of improved healing:
- Increased epidermal thickness: The epidermis was 43% thicker in the WPED-treated group compared to the sham group, and 72% thicker than the control group.
- Enhanced collagen production: Masson’s trichrome staining showed increased collagen intensity in the WPED-treated wounds.
- Improved angiogenesis: CD31 staining, a marker for blood vessels, was notably higher in the WPED-treated group.
- Reduced inflammation: The ratio of pro-inflammatory M1 macrophages to anti-inflammatory M2 macrophages was significantly lower in the WPED-treated wounds, indicating a shift from the inflammatory phase to the proliferative and remodelling phases of wound healing.
Versatile and robust design
One of the key advantages of the WPED is its versatility and robustness. The researchers demonstrated that the dressing can function effectively under a wide range of conditions, including extreme temperatures (-15°C to 45°C), varying humidity levels, and high external pressures (up to 450 kPa).
The team also developed a version of the WPED with kirigami-inspired 3D morphable electrodes, allowing the dressing to conform to wounds with complex shapes and depths. This adaptability could make the WPED suitable for treating a variety of wound types in different body locations.
Potential impact and future directions
The WPED represents a significant advance in wound care technology, offering a low-cost, easy-to-use alternative to more complex and expensive treatments. At an estimated cost of just $1 per dressing, the WPED could make advanced wound care more accessible, particularly in resource-limited settings.
“Next steps for us include additional work to fine-tune our ability to reduce fluctuations in the electric field and extend the duration of the field. We are also moving forward with additional testing that will get us closer to clinical trials and, ultimately, practical use that can help people,” said Bandodkar.
The researchers suggest that future studies should investigate the efficacy of the WPED in larger animal models that more closely resemble human wound healing, such as porcine models. Additionally, they propose exploring the device’s potential to prevent infection, as previous studies have shown that electrical stimulation can disrupt biofilms and inhibit bacterial growth in wounds.
Reference:
- Wang, Y., Joshi, S., Yuk, H., Yokota, H., Ahn, S., Rogers, J. A., & Yeo, W. H. (2024). Water-powered, electronics-free dressings that electrically stimulate wounds for rapid wound closure. Science Advances, 10(32).
https://doi.org/10.1126/sciadv.ado7538

