Novel gene therapy restores vision in mice by preventing Prox1 protein transfer
Korean researchers have developed a breakthrough gene therapy that restores vision loss caused by retinal degeneration. The therapy works by neutralising a protein called Prox1 that suppresses retinal regeneration. When the Prox1 protein is blocked, Müller glial cells in the retina can reprogram into stem-like cells that regenerate damaged neurons, restoring visual function.
(From left) Ph.D. candidate Museong Kim, Professor Jin Woo Kim, and Dr. Eun Jung Lee of KAIST Department of Biological Sciences
KAIST scientists discover key inhibitor of retinal regeneration
Researchers at the Korea Advanced Institute of Science and Technology (KAIST) have successfully developed a new treatment approach that can restore vision through retinal nerve regeneration, offering hope to millions suffering from retinal degenerative diseases.
The groundbreaking study, published in Nature Communications on 25 March 2025, demonstrates how blocking a protein called Prox1 allows the mammalian retina to regenerate neurons, something previously thought impossible in mammals.
“We are about completing the optimisation of the PROX1-neutralising antibody (CLZ001) and move to preclinical studies before administering it to retinal disease patients. Our goal is to provide a solution for patients at risk of blindness who currently lack proper treatment options,” said Dr Eun Jung Lee, study co-first author and researcher at Celliaz Inc., a biotech startup founded by Professor Jin Woo Kim’s research lab at KAIST.
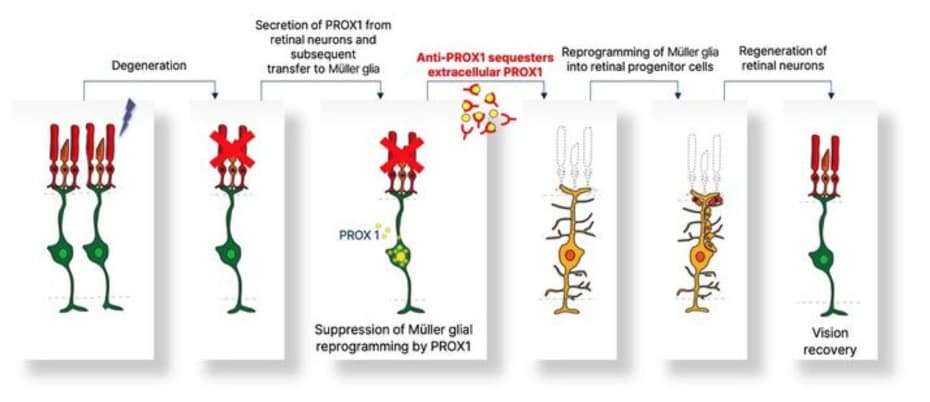
Figure 1. Schematic diagram of the mechanism of retinal regeneration through inhibition of PROX1 migration. PROX1 protein secreted from retinal damaged retinal neurons transfers to Müllerglia and inhibits dedifferentiation into neural progenitor cells and neural regeneration. When PROX1 is captured outside the cells by an antibody against PROX1 and its transfer to Müllerglia is interfered, dedifferentiation of Müllerglia cells and retinal regeneration processes are resumed, restoring visual function. © KAIST Laboratory of Neural Development and Regeneration
The breakthrough: Blocking Prox1 protein transfer
The research team identified Prox1 (prospero homeobox 1) as a critical factor that prevents retinal regeneration in mammals. Unlike cold-blooded animals such as fish and amphibians, which can regenerate damaged retinas, mammals lose this ability, leading to permanent vision loss from conditions like retinitis pigmentosa and macular degeneration.
The key discovery was that Prox1 protein accumulates in Müller glial cells (MG) of damaged mammalian retinas but is absent in the regenerative MG cells of fish. Notably, the researchers found that Prox1 in mammalian MG isn’t produced internally but is transferred from surrounding neurons that fail to degrade the protein.
By developing an antibody that captures Prox1 before it reaches MG cells, the team was able to restore their regenerative capacity. When delivered via adeno-associated virus (AAV) to mouse models of retinitis pigmentosa, the therapy enabled sustained retinal regeneration and vision restoration that lasted over six months.
How the therapy works at the molecular level
“Blocking Prox1 alone may be insufficient to fully restore regenerative capacity in the mammalian retina to that of zebrafish retina,” the authors write in their paper. “Additional events, such as Notch inhibition post-reprogramming and sustained Yap/Taz activation, may be needed to promote robust MGPC proliferation.”
When Prox1 is neutralised, Müller glial cells in the damaged retina undergo reprogramming into retinal progenitor cells. These cells can then divide and differentiate into various retinal neurons, including photoreceptors that are essential for vision. The researchers used gene sequencing to identify the molecular changes occurring during this reprogramming process, revealing upregulation of neurogenic factors and cell cycle genes.
“Prox1-depleted MG of the injured mouse retina may be reprogrammed into RPCs through mechanisms independent of Ascl1 induction, possibly involving other proneural transcription factors,” the authors explain in the paper.
Promising results in two different mouse models
The research team tested their approach in two mouse models of retinitis pigmentosa: an early-onset model (Pde6brd10/rd10) and a late-onset model (Rp1tvrm64/tvrm64).
In the early-onset model, the therapy delayed vision loss significantly, though the effect was temporary due to the rapid progression of retinal degeneration. The therapy was more effective in the late-onset model, where mice maintained normal visual acuity while untreated mice showed gradual vision decline.
Remarkably, in the late-onset model, even mice that had already begun to lose vision showed recovery of visual function within one month of treatment, maintaining this improvement for six months.
Future clinical applications
The retinal regeneration-inducing therapy is currently being developed by Celliaz Inc. for application in various degenerative retinal diseases that currently lack effective treatments. The company aims to begin clinical trials by 2028.
“The blocking PROX1 transfer could be a potent therapeutic strategy for retinal degenerative diseases, if exogenous PROX1 also plays the suppressive roles in human retinal regeneration,” the researchers note in their paper. They have already found evidence that PROX1 accumulates in Müller glial cells of human retinitis pigmentosa patients but not in healthy donor retinas, suggesting the mechanism is relevant to human disease.
This research was supported by research funds from Korean National Research Foundation (NRF) and the Korea Drug Development Foundation (KDDF).
From bench to bedside: Overcoming remaining challenges
The researchers acknowledge that several challenges remain before this therapy can be translated to human patients. The current vector system used for delivering the antibody gene showed silencing of expression after six months, limiting the duration of therapeutic effect. Redesigning the promoter could improve long-term efficacy.
Additionally, while the therapy showed significant benefits, it did not fully restore retinal structure and function to normal levels. Further refinements, including combinations with other regenerative approaches, may be needed to maximise therapeutic outcomes.
If successful in human trials, this innovative approach could revolutionise treatment for retinal degenerative diseases, offering the first therapy capable of restoring lost vision rather than merely slowing disease progression.
Reference:
Lee, E. J., Kim, M., Park, S., et. al. (2025). Restoration of retinal regenerative potential of Müller glia by disrupting intercellular Prox1 transfer. Nature Communications, 16, 2928. https://doi.org/10.1038/s41467-025-58290-8 (Open Access)

