Novel brain-adhesive sensor enables precise epilepsy treatment monitoring
South Korean researchers have developed a soft, adhesive brain sensor that could significantly improve the effectiveness of ultrasound-based epilepsy treatments by enabling accurate, real-time monitoring of brain activity during stimulation.
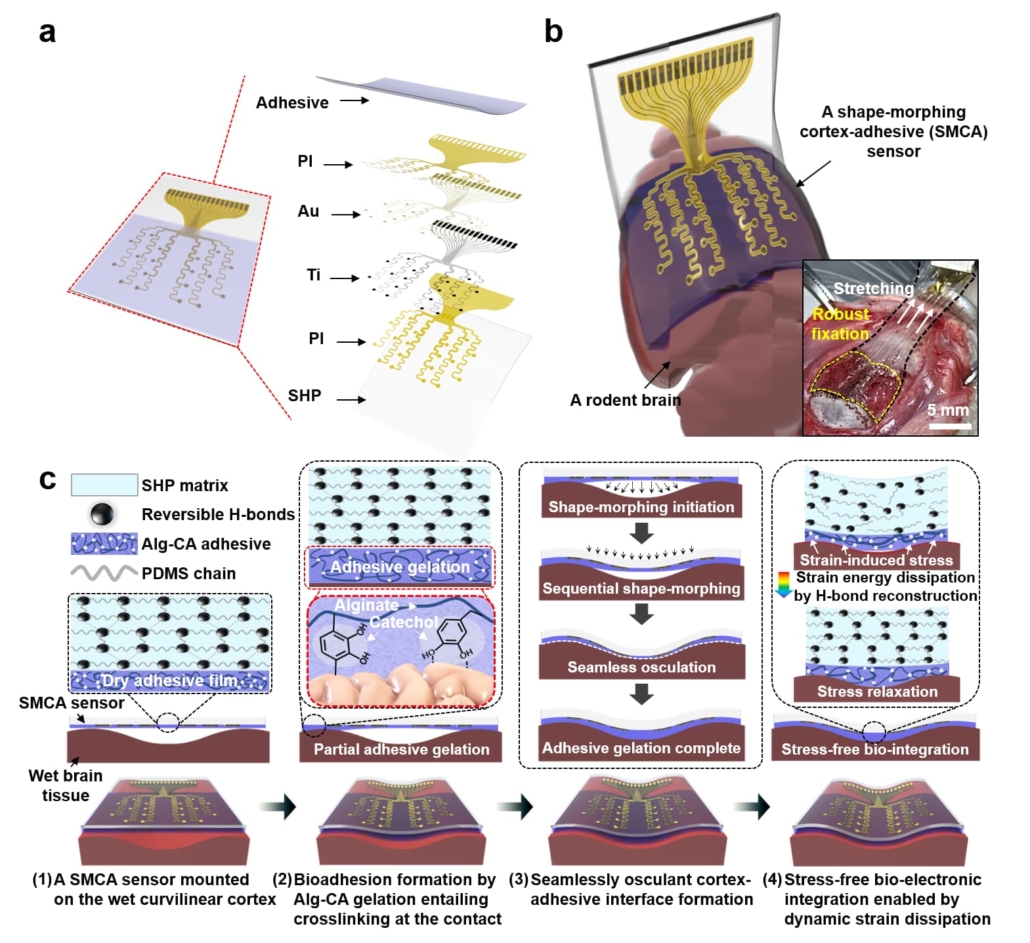
Overview and operation principle of a shape-morphing cortex-adhesive (SMCA) sensorA. A schematic illustration for exploded view of a SMCA sensor. B. A schematic illustration for a SMCA sensor mounted conformally on a rodent’s brain tissue. (Inset) a photoimage illustrating robust tissue adhesion of a SMCA sensor on a rat’s cortex under shear strain. C. Schematic illustrations of sequential brain-interfacing steps of the SMCA sensor for explaining the tissue-adhesive shape-morphing mechanism.
The breakthrough, which addresses a longstanding challenge in neuromodulation therapy, could particularly benefit patients with drug-resistant epilepsy who currently have limited treatment options.
Epilepsy affects more than 65 million people globally, with approximately 20-30% of patients experiencing intractable epilepsy that does not respond to conventional anticonvulsant medications. Whilst surgical resection of affected brain tissue remains an option for these patients, its invasive nature and associated risks have driven research into alternative treatments.
Transcranial focused ultrasound (tFUS) neurostimulation has emerged as a promising non-invasive approach for treating epilepsy. However, its effectiveness has been limited by the inability to accurately monitor brain activity during treatment, largely due to interference from the ultrasound waves and poor contact between monitoring devices and brain tissue.
Technical innovation
The research team, led by Professor Donghee Son and Professor Mikyung Shin from the Center for Neuroscience Imaging Research at the Institute for Basic Science (IBS), has developed what they term a Shape-Morphing Cortical-Adhesive (SMCA) sensor. This device incorporates two key innovations: a catechol-conjugated alginate hydrogel layer that forms strong bonds with brain tissue, and a self-healing polymer substrate that becomes pliable at body temperature, allowing it to conform to the brain’s irregular surface.
Clinical testing and outcomes
The researchers conducted both ex vivo and in vivo testing of the SMCA sensor, comparing its performance against conventional devices. In experiments using a rat model of epilepsy, the sensor demonstrated superior ability to record brain activity during tFUS stimulation without signal interference.
The team successfully implemented a closed-loop seizure control system, where the SMCA sensor detected early seizure activity and automatically triggered appropriate tFUS treatment responses. This represents a significant advance towards personalised, adaptive epilepsy treatment.
Future directions
Professor Son emphasised the broader implications of their work: “Through our study on the brain-adhesive soft bioelectronics platform, we have overcome a major challenge in the field of brain interfaces by achieving high-quality electrocorticography coupled with focused ultrasound stimulation without artifact interference.”
Dr Hyungmin Kim from the Korea Institute of Science and Technology, who collaborated on the research, highlighted the potential for further development: “We achieved early detection of seizure activity via ECoG, enabling the prevention of seizures. Additionally, we implemented real-time feedback on the effects of ultrasound stimulation, which allowed for the application of personalized stimulation protocols.”
The research team plans to enhance the SMCA sensor platform by improving its shape-morphing and cortex-adhesive capabilities, developing more sophisticated integrated microelectrodes, and implementing advanced closed-loop operational algorithms.
Clinical implications
The development of this technology could particularly benefit patients with intractable epilepsy who have exhausted other treatment options. The ability to precisely monitor brain activity during tFUS treatment could enable more effective, personalised therapeutic approaches whilst minimising potential side effects.
Reference:
Lee, S., Kum, J., Kim, S., et. al. (2024). A shape-morphing cortex-adhesive sensor for closed-loop transcranial ultrasound neurostimulation. Nature Electronics. https://doi.org/10.1038/s41928-024-01240-x

