Newly engineered human brain organoids reveal myelin production and repair processes
Researchers have engineered sophisticated human brain organoids that faithfully model myelin production and repair processes. The groundbreaking model integrates immune cells called microglia, revealing their essential role in remyelination and providing a powerful new platform for testing potential multiple sclerosis therapies.
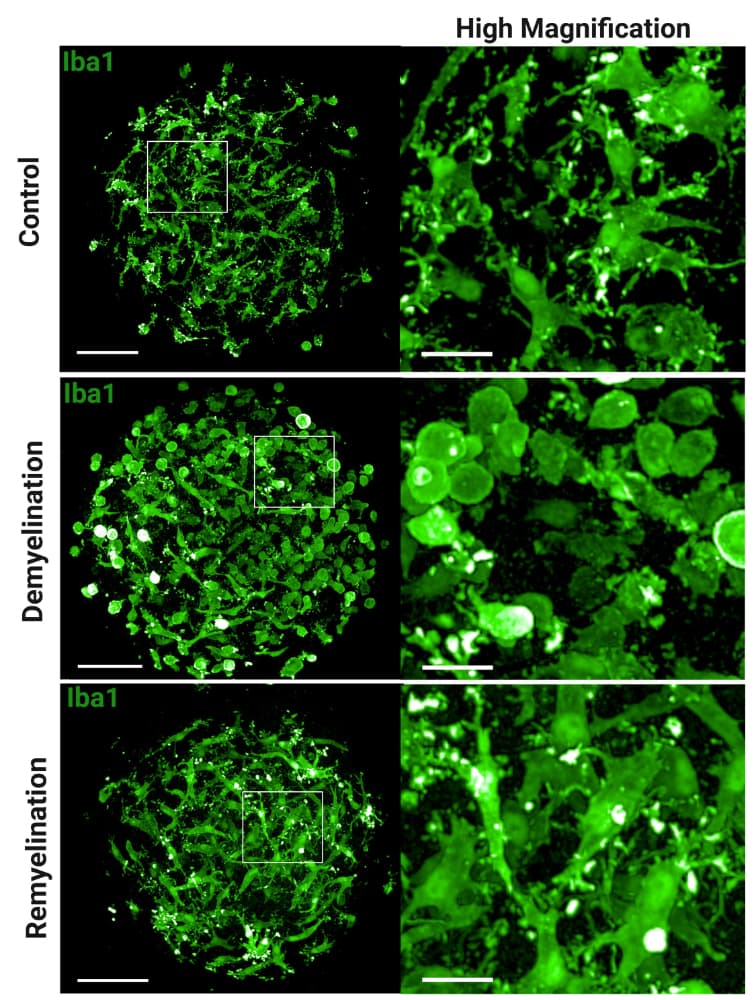
Microglia integrated in human brain organoids respond to toxin-induced demyelination with morphological changes. Representative immunofluorescence images show microglia (IBA1, green) in a human brain organoid model. Panels show the response to control conditions, demyelination (induced by toxin), and remyelination, respectively. Magnified insets an the right highlight the detailed morphological changes of microglia in each condition. © Lange et al., Sci. Trans. Med. 17, eadp7047 (2025).
Scientists have developed an advanced human brain organoid system that could transform our understanding of myelin repair and accelerate the development of treatments for demyelinating diseases such as multiple sclerosis. The research, published in Science Translational Medicine on 10 September 2025, demonstrates how integrating immune cells called microglia into laboratory-grown brain tissue creates a more accurate model of human myelin biology.
Enhanced brain organoid model bridges research gap
The new organoids mark a notable advance in the study of remyelination and demyelination, and could serve as useful research tools to investigate demyelinating disorders such as multiple sclerosis. Led by researchers at F. Hoffmann-La Roche, the study addresses a critical limitation in current research approaches that rely heavily on animal models.
Myelin acts as an insulating coating that protects neurons’ delicate axons and facilitates the transmission of nerve impulses. Many neurological disorders feature the loss and degradation of myelin, including autoimmune diseases, neurodegenerative conditions, and autism and bipolar disorder.
The research team, headed by Simona Lange and colleagues, built upon previous brain organoid technologies to create what they term myelinated human brain organoids with integrated microglia (MHBOs +MG). These sophisticated models contain neurons, oligodendrocytes (the cells that produce myelin), astrocytes, and crucially, microglia – the brain’s resident immune cells.
Microglia prove essential for myelin regeneration
The study’s most significant finding was the indispensable role of microglia in myelin repair processes. When researchers induced demyelination using toxins, only organoids containing microglia showed the characteristic pattern of myelin loss followed by spontaneous regeneration.
Using their organoids, Lange et al. confirmed that microglia were essential for remyelination after myelin damage from exposure to toxins and were also necessary for the myelin-regenerating effects of the drug clemastine and the experimental compounds XAV939 and BQ3020.
The researchers observed that microglia underwent dramatic morphological changes during the demyelination process, transforming from their normal ramified appearance to an amoeboid shape characteristic of activated immune cells. Electron microscopy revealed microglia containing numerous inclusions, potentially representing degenerating myelin that the cells had engulfed and were clearing away.
Advanced molecular analysis reveals repair mechanisms
Through comprehensive transcriptomic and proteomic analyses, the team identified key molecular signatures associated with demyelination and recovery. During demyelination, genes involved in cholesterol metabolism – crucial for myelin production – were significantly downregulated. Conversely, during remyelination, these same genes showed marked upregulation, particularly in organoids containing microglia.
The molecular analysis also revealed increased expression of microglia activation markers such as legumain (LGMN) and triggering receptor expressed on myeloid cells 2 (TREM2) during demyelination, followed by their downregulation during recovery. This pattern suggests a shift from inflammatory to reparative microglial phenotypes.
Therapeutic compound testing validates model utility
To demonstrate the clinical relevance of their system, researchers tested three compounds known to promote remyelination: clemastine fumarate, XAV939, and BQ3020. All three significantly enhanced remyelination in organoids containing microglia, but showed no effect in organoids lacking these immune cells.
Clemastine fumarate, an antihistamine that has shown promise in clinical trials for multiple sclerosis, was particularly effective. Transcriptomic analyses revealed that clemastine treatment led to pronounced increases in expression of genes related to cholesterol biosynthesis, suggesting enhanced oligodendrocyte differentiation and myelin formation.
The researchers validated their findings using mouse cerebellar slice cultures, where clemastine and XAV939 treatments also significantly increased myelination indices, confirming the translational potential of their human organoid model.
Clinical implications and future directions
The development represents a significant advance in creating more physiologically relevant models of human brain function and disease. The new organoid “represents a relevant system for studying human diseases and exhibits the potential to accelerate the development of new treatments for demyelinating disorders,” the authors conclude.
The model’s ability to recapitulate key aspects of human myelin biology, including the critical role of immune cells in repair processes, addresses longstanding limitations of animal models that may not fully translate to human pathophysiology.
Study limitations and future development
The researchers acknowledge several limitations of their current model. The organoids lack the complex three-dimensional architecture and cellular diversity of the human brain, including vascular components and a blood-brain barrier. Additionally, the model cannot fully capture age-related phenomena due to the relative immaturity of the organoid tissue.
Despite these constraints, the research provides a sophisticated new platform for studying human-specific aspects of myelination and testing potential therapeutic interventions in a controlled laboratory setting that more closely mimics natural human brain environments than previous models.
Reference
Lange, S., Ebeling, M., Loye, A., et. al. (2025). Human myelinated brain organoids with integrated microglia as a model for myelin repair and remyelinating therapies. Science Translational Medicine, 17(eadp7047). https://doi.org/10.1126/scitranslmed.adp7047

