New catheter aims to reduce catheter-associated UTIs and improve patient quality of life
A novel indwelling catheter called cymactive 2.0R has received CE marking in Europe, allowing it to be marketed for men experiencing chronic, non-neurogenic urinary retention.
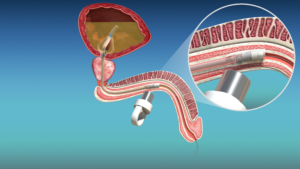
The catheter, made by UK-based Ingenion Medical, has an innovative design aimed at mimicking natural urination while potentially reducing catheter-associated urinary tract infections (CAUTIs).
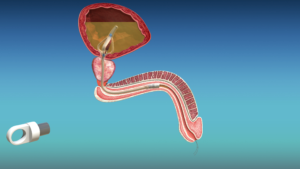
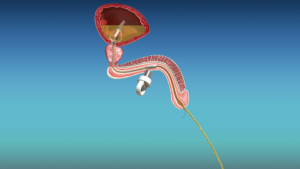
Unlike standard Foley catheters which require external drainage bags, cymactive uses an internal “Malecot” anchor with four wings that sits in the bladder. A valve called the UroValve in the catheter portion within the urethra can be opened using an external magnet, allowing the bladder to empty naturally through urination.
“cymactive has been designed with the patient in mind to provide a better solution compared to both Foley-type catheters and intermittent catheters,” said Edward Cappabianca, Ingenion Medical CEO. Key intended benefits include improving quality of life, reducing CAUTI risk, promoting natural bladder function, and reducing environmental waste from disposable external drainage bags.
Approved for up to 30 days of continuous use
The catheter is approved for up to 30 days of continuous use. Its discreet design has just two slender removal threads extending from the penis, combining the stability of an indwelling Foley with the autonomy of intermittent self-catheterization.
CAUTIs are a major issue, affecting 15-25% of hospitalized patients and 5% of nursing home residents in Europe. Frequent re-catheterization required with intermittent catheters is a common infection source. By reducing CAUTI rates, cymactive could lower antibiotic use, hospital readmissions, and healthcare costs.
“There is a significant unmet need for men relating to intervention following urinary retention. Potentially, this device will prove to be a very effective alternative solution,” commented Chris Chapple, Emeritus Urological Surgeon at Sheffield Teaching Hospitals NHS Trust.
Ingenion plans to develop the catheter’s core valve technology for additional indications like women’s incontinence and retention. The global urinary catheter market was valued at $5.5 billion in 2023.
- For more information, visit: ingenionmedical.co.uk.