Molecular imaging biomarker predicts response to CAR T cell therapy
A new PET imaging agent, 18F-AlF-FAPI-74, has been found to effectively monitor and predict treatment response of an up-and-coming cancer therapy. This non-invasive imaging approach has the potential to help inform important clinical decision-making early in the course of treatment – including re-dosing of therapy, dose optimization, change of therapeutic course, and many more – to optimize patient outcomes. The research was presented at the Society of Nuclear Medicine and Molecular Imaging 2022 Annual Meeting.
A member of the research team, Iris Lee, PhD candidate at the University of Pennsylvania, noted: “In the current era of precision medicine where cancer therapies can be tailored based on the presence or absence of a biomarker, molecular imaging tools have the potential to add value to clinical care by helping to accurately monitor the target of interest and stratify patients for certain therapy based on the target expression level.”
Chimeric antigen receptor T cell (CAR T) therapy is a treatment that involves removing T cells from a patient’s body, engineering them in a lab, and then returning the T cells to the body to fight the specified disease. This type of therapy has had marked success in treating blood cancers but has not been as effective in treating solid tumours.
“Our understanding of why CAR T therapies are not as effective in solid tumours could be advanced by measuring CAR T-targeted antigens, such as the fibroblast activation protein (FAP), which is overproduced in many types of solid tumours,” said Lee. “In this study, we used 18F-AlF-FAPI-74 PET to image FAP with the goal of providing information that could aid in the development and optimization of FAP CAR T cell treatment for solid tumours.”
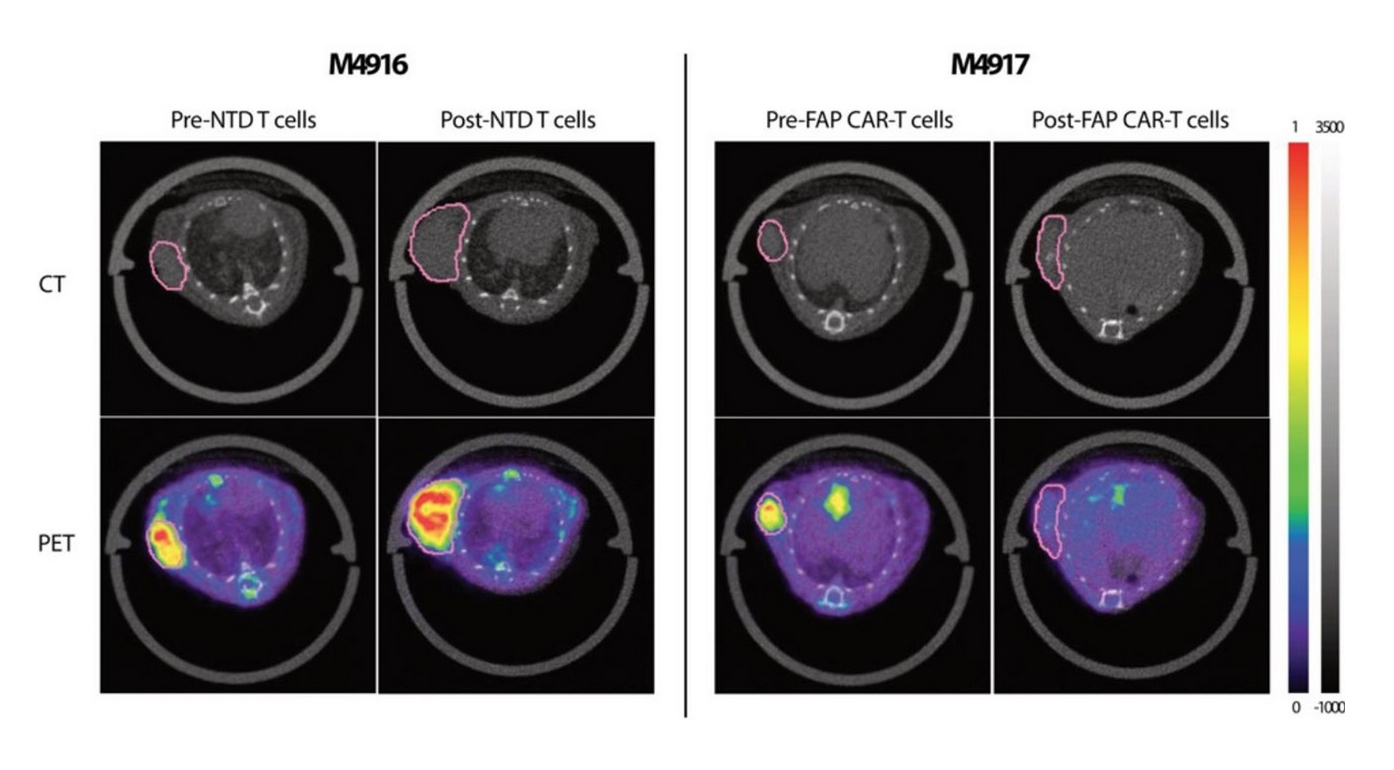
Several experiments were conducted as part of the study. In the first, an in vitro cell uptake experiment was performed to test the binding of the 18F-AlF-FAPI-74 tracer in FAP-expressing cells. Next, the tracer uptake was assessed in two different tumour xenograft models (mesothelioma and adenocarcinoma). Finally, researchers evaluated whether 18F-AlF-FAPI-74 could detect FAP clearance as a way to monitor response to FAP CAR T cell treatment.
The cell uptake experiment demonstrated highly specific binding of 18F-AlF-FAPI-74 to FAP. For both xenograft models, a significant increase in 18F-AlF-FAPI-74 uptake in the tumour was noted relative to background tissues (e.g. muscle and blood). 18F-AlF-FAPI-74 PET was also able to successfully detect and image clearance of FAP+ cells from the tumour following an FAP CAR T cell treatment.
“Our work highlights the potential role of 18F-AlF-FAPI-74 as a predictive and pharmacodynamic imaging biomarker to guide patient selection and monitor therapeutic response to FAP-targeted therapies,” Lee commented.
Representative 18F-AlF-FAPI-74 PET/CT images showed statistical differences in tracer uptake between non-transduced (NTD) T cell-treated (left, M4916) and FAP CAR T cell-treated (right, M4917) animals.