Medtronic’s MiniMed 780G closed loop system for Type 1 diabetes management receives CE Mark
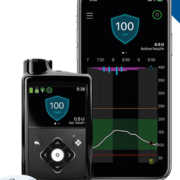
Medtronic received CE Marking of its MiniMed™ 780G system, a next generation closed loop insulin pump system for the treatment of type 1 diabetes in people age 7 to 80 years.Leveraging the company’s advanced SmartGuard™ algorithm, the system automates the delivery of both basal insulin and correction boluses every five minutes to help people with diabetes avoid highs and lows with greater ease.
The MiniMed 780G system enables the personalization of glucose goals with an adjustable target setting as low as 100 mg/dL (5.5 mmol/L) – lower than any other advanced hybrid closed loop system – and is designed to help stabilize blood sugar levels and further improve glucose control.
“We wanted to design a system that further simplifies diabetes management and adapts to people’s life with the goal of enhancing their experience in a seamless way,” said Sean Salmon, Executive Vice President and President of the Diabetes Group at Medtronic. “We know it can be challenging to have to calculate carbohydrate intake before every snack or meal on a daily basis to ensure the right amount of insulin is dosed. With this system, users will have an extra layer of coverage for those times they miscalculate their carbs or forget to pre-bolus with an algorithm that automatically corrects for high glucose when needed. We want to help people spend more time living their life and less time worrying about their diabetes management – we’re confident this system delivers on that important goal.”
In addition to the automated algorithm which includes technology from DreaMed Diabetes, the MiniMed 780G system is easy to use with little input from the user. With the addition of Bluetooth connectivity, the MiniMed 780G system will enable users and their care partners to see real-time glucose data and trends on compatible iOS and Android smartphones via apps. Additionally, healthcare providers will find that managing patients on the system is simple as there are only a few settings that need adjustment to enable optimal use of the technology.
The system is expected to begin shipping this Autumn in select countries in Europe. In the United States, the MiniMed 780G system is investigational use only and not approved for sale.
For more information, visit: www.medtronicdiabetes.com