Medtronic’s Hugo Robotic-Assisted Surgery system receives CE Mark
Medtronic has received CE Mark for the Hugo robotic-assisted surgery (RAS) system, authorizing the sale of the system in Europe. CE Mark approval is for urologic and gynaeco- logic procedures, which make up about half of all robotic procedures performed today.
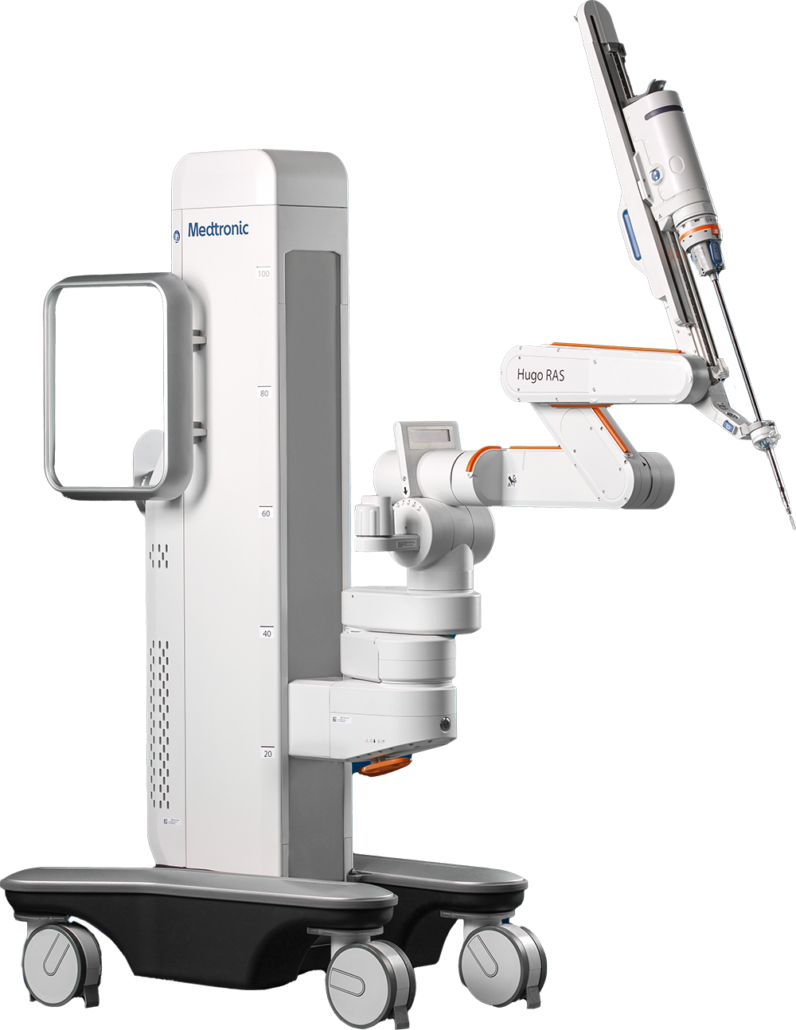
The Hugo RAS system is a modular, multiquadrant platform designed for a broad range of surgical procedures. It combines wristed instruments, 3D visualization, and a cloud-based surgical video capture option in Touch Surgery Enterprise with dedicated support teams specializing in robotics program optimization, service, and training.
“This day has been a long time coming, not just for Medtronic, but for the surgeons and hospital leaders who have partnered with us on this journey to bring the benefits of robotic-assisted surgery to more patients around the world. We know our solution is more meaningful because of their insights,” said Megan Rosengarten, president of the Surgical Robotics business, which is part of the Medical Surgical Portfolio at Medtronic. “With the Hugo RAS system in our European customers’ hands and our dedicated team by their side, together we will redefine what is possible in robotic-assisted surgery.”
The Hugo RAS system was designed to address the historic cost and utilization barriers that have stifled robotic surgery adoption for two decades. Globally, about 3% of surgeries are performed robotically, despite offering patients the benefits of minimally invasive surgery — fewer complications, shorter hospital stays, and faster return to normal activities. In Western Europe, about 2% of procedures are done robotically while the majority, approximately 65%, are open surgery. The remainder are traditional minimally invasive surgery.
“Robotics and artificial intelligence are the undeniable future of healthcare, with incre- dible potential to not only advance patient care, but increase access to these benefits,” said Rob ten Hoedt, executive vice president and president of the Europe, Middle East, and Africa (EMEA) region at Medtronic. “The Hugo RAS system builds on our leadership in minimally invasive surgery, and we’re thrilled to provide hospitals across Europe a robotic-assisted surgery system that is thoughtfully designed to meet their needs. We’ve had strong interest from leading surgical centres across Europe and expect to move quickly with multiple installations in several countries.”
These hospitals will be the first in Europe to join Medtronic’s Partners in Possibility Program, a group of pioneering institutions that will be among the first in the world to use the Hugo RAS system and participate in the global patient registry. Clinicians from these hospitals will participate in hands-on training at Medtronic Surgical Robotics Experience Centers, including two flagship sites operated in partnership with the ORSI Academy in Ghent, Belgium, and IRCAD in Strasbourg, France.
CE Mark approval comes on the heels of major milestones in the Hugo RAS system global launch, including the first urological and gynaecological procedures, which took place in Latin America and India earlier this year and marked the start of the Hugo RAS system global patient registry.
In the U.S., the Hugo RAS system is an investigational device not for sale. Touch Surgery Enterprise is not intended to direct surgery, or aid in diagnosis or treatment of a disease or condition.