Medovate expands SAFIRA product range with further CE Mark approvals
Medovate has successfully secured additional CE Mark approvals for its SAFIRA (SAFer Injection for Regional Anaesthesia) technology to include an NRFit syringe and a Palm Operator
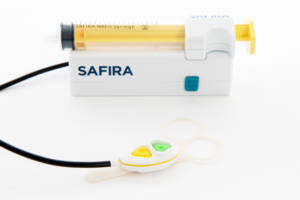
Within the last year, Medovate has achieved CE Mark regulatory approval covering five devices in its SAFIRA range: infusion driver, foot pedal, luer syringe, NRFit syringe and palm operator. These latest product range additions are intended to further improve patient safety and offer greater control to anaesthetists.
The NRFit syringe provides a second option to the universal Luer connection syringes with which the system was originally introduced. It is recognised that Luer connectors carry the potential for medical tubing misconnections, which can lead to incidences of wrong route administration of medicines and gases. The NHS issued a Patient Safety Alert in 2019 to recommend and support the safe transition from the Luer connector to NRFit for the delivery of regional anaesthesia
With the provision of a new Palm Operator, Medovate hopes to offer anaesthetists more versatility and choice. The palm operator uses the same colour coding as the original foot pedal operator, which remains a key part of the product range for the infusion and aspiration of anaesthetics. The palm operator is ergonomically designed to comfortably fit under a surgical glove, within the palm of a hand. The addition of a hand operator product to the SAFIRA range offers regional anaesthetists greater choice when using SAFIRA.
Developed with the NHS to promote safer injection during peripheral nerve block procedures, SAFIRA transforms regional anaesthesia into a one-person procedure, giving the anaesthetist full control of the injection at all times.
It also includes a built-in safety mechanism to limit injection pressure helping to reduce the risk of nerve damage and promote patient safety.