Major advance in human in vitro gametogenesis achieved
A groundbreaking study published in Nature [1] has reported a significant milestone in the field of human in vitro gametogenesis (IVG), the process of generating mature gametes (sperm and eggs) from pluripotent stem cells in a culture dish.
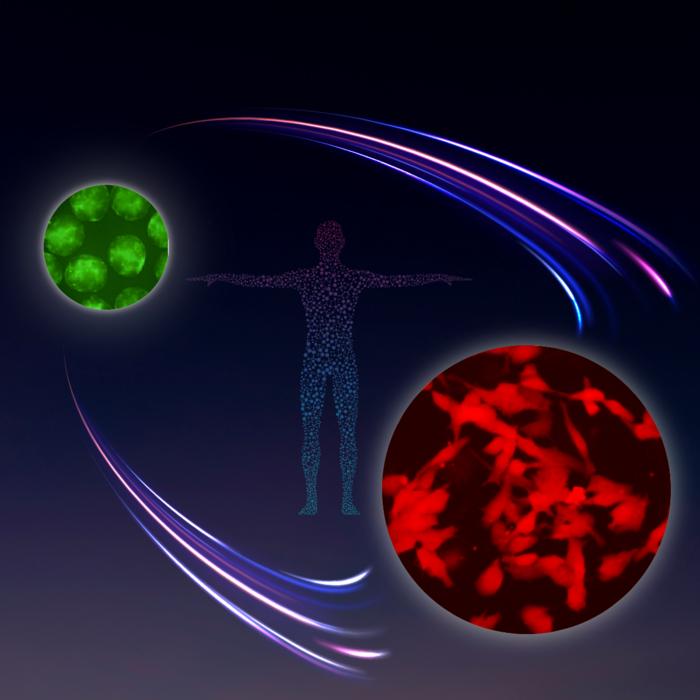
Image inspired by NASA’s Apollo Program, representing the successful in vitro germ cell differentiation from TFAP2C-EGFP +ve human primordial germ cell-like cells (hPGCLCs; labelled in green) to DAZL-tdTomato +ve human mitotic pro-spermatogonia (labelled in red).
This achievement brings us one step closer to the potential treatment of all forms of infertility through IVG-based therapies.
Infertility, as defined by the American Society for Reproductive Medicine (ASRM), is a disease characterized by the inability to achieve a successful pregnancy without medical intervention. Globally, it affects approximately one in six individuals worldwide, according to the World Health Organization (WHO). While assisted reproductive technologies (ARTs) like in vitro fertilization (IVF) have revolutionized the treatment of certain forms of infertility, not all cases can be addressed using existing strategies.
Overcoming the recapitulation of epigenetic reprogramming
The research was conducted by a team led by Dr Mitinori Saitou at the Institute for the Advanced Study of Human Biology (WPI-ASHBi) in Kyoto University. Their study focused on overcoming a major challenge in human IVG research: the recapitulation of epigenetic reprogramming, a hallmark event in which the inherited “memory” of cells, present on its DNA, is reset or erased, allowing for proper germ cell differentiation.
Previous efforts by Saitou’s team and others had successfully generated human primordial germ cell-like cells (hPGCLCs) from pluripotent stem cells in vitro, mimicking the early stages of germ cell development. However, these hPGCLCs were unable to undergo epigenetic reprogramming and subsequent differentiation into precursors of mature gametes, known as mitotic pro-spermatogonia and pro-oogonia.
The crucial role of bone morphogenetic protein
In their new study, Saitou and colleagues conducted a cell culture-based screen to identify signalling molecules that could drive epigenetic reprogramming and differentiation of hPGCLCs. Surprisingly, they found that the developmental signalling molecule bone morphogenetic protein (BMP) played a crucial role in this process.
“Indeed, considering that BMP signalling already has an established role in germ cell specification, it was highly unexpected that it also drives hPGCLC epigenetic reprogramming,” said Saitou.
The resulting hPGCLC-derived mitotic pro-spermatogonia and oogonia exhibited remarkable similarities in gene expression and epigenetic profiles to actual human primordial germ cell differentiation in vivo. Moreover, these cells underwent extensive amplification, with over 10 billion-fold expansion, enabling near-indefinite propagation in culture.
“Our approach enables near-indefinite amplification of mitotic pro-spermatogonia and oogonia in culture, and we now also have the ability to store and re-expand these cells as needed,” said Saitou.
The authors also provided insights into the potential mechanisms by which BMP signalling facilitates epigenetic reprogramming and hPGCLC differentiation. “BMP (signalling) appears to be attenuating the MAPK/ERK (mitogen-activated protein kinase/extracellular-regulated kinase) signalling pathway and both the de novo and maintenance activities of DNMT (DNA methyltransferase), but further investigation will be necessary to determine the precise mechanism and whether this is direct or indirect,” explained Saitou.
A fundamental advance in understanding epigenetic reprogramming
This study represents a fundamental advance in our understanding of human biology and the principles behind epigenetic reprogramming in humans. It is a true milestone in human IVG research, bringing us closer to the potential translation of IVG into reproductive medicine.
“Although many challenges remain and the path will certainly be long, especially when considering the ethical, legal, and social implications associated with the clinical application of human IVG, nevertheless, we have now made one significant leap forward towards the potential translation of IVG into reproductive medicine,” said Saitou.
The findings reported in this Nature study have paved the way for further progress in the field of human IVG, offering hope for the development of new treatments for infertility.
Reference:
1. Murase, Y., Yokogawa, R., Yabuta, Y. et al. In vitro reconstitution of epigenetic reprogramming in the human germ line. Nature (2024).
https://doi.org/10.1038/s41586-024-07526-6

