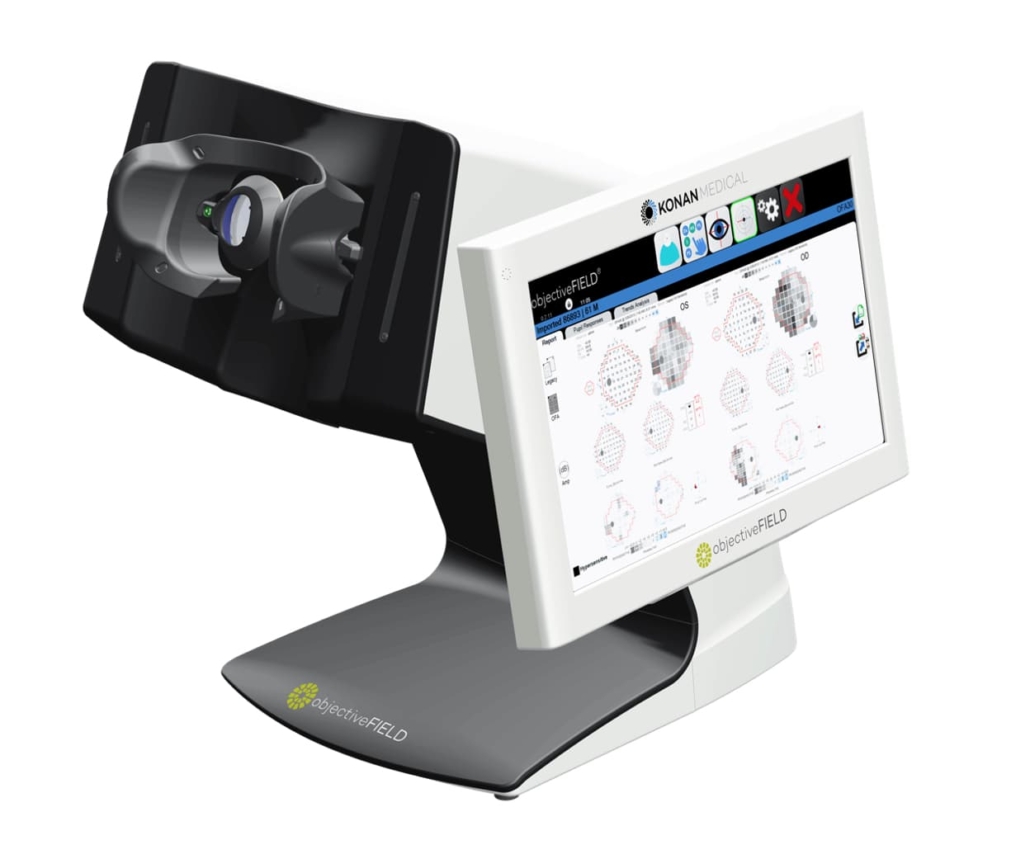
Konan Medical receives CE mark for objective visual field analyser
Konan Medical USA has secured CE marking under the European Medical Device Regulation for its objectiveFIELD Analyzer (OFA), enabling distribution across the European Union. The device represents the first FDA 510(k)-cleared objective perimeter that assesses visual field abnormalities through pupillary response measurement rather than subjective patient input
Pupillometry-based assessment technology
The OFA employs bilateral video cameras operating under infrared conditions to capture pupillary responses to spatially resolved stimuli. Unlike standard automated perimetry (SAP), which relies on patient-initiated button responses to white-on-white light flashes, the system derives retinal sensitivity from pupil response amplitude whilst simultaneously measuring constriction delay and latency – parameters unavailable through conventional SAP devices.
The technology’s dual-axis capability differentiates it from half-axis SAP systems. Whilst SAP exclusively detects reduced sensitivity defects, OFA identifies both reduced and high-sensitivity abnormalities without additional testing time. Test protocols complete bilateral assessment in as little as 90 seconds, with results presented in formats familiar to clinical practitioners.
Regulatory approval and clinical applications
“We are thrilled to have achieved CE Marking for the OFA. It is a testament to our team’s dedication to innovation and developing novel medical technology,” said Simon Gordon, co-chief executive officer of Konan Medical USA.
The CE marking approval followed rigorous assessment confirming conformity with MDR Regulation EU 2017/745, including comprehensive review of clinical data, technical documentation, and manufacturing processes. Konan Medical USA’s quality system holds ISO 13485:2016 MDSAP certification.
Enhanced clinical efficiency
Co-CEO Dale Sadlik emphasised the technology’s potential to expand testing accessibility: “This approval is pivotal for expanding access to new, objective visual-function testing, which we believe will augment current standard of care and enable testing patients that are too young for traditional subjective methods.”
Clinical interest has concentrated on applications in diabetic retinal disease and paediatric populations, where objective measurement eliminates reliance on patient response capability. The device is currently available in the USA, Canada, Japan, and select international markets, with European distribution commencing October 2025.
The OFA’s development is supported by 39 peer-reviewed clinical and scientific publications documenting its performance and clinical utility across multiple patient populations.
- For more information, visit: https://konanmedical.com/objectivefield