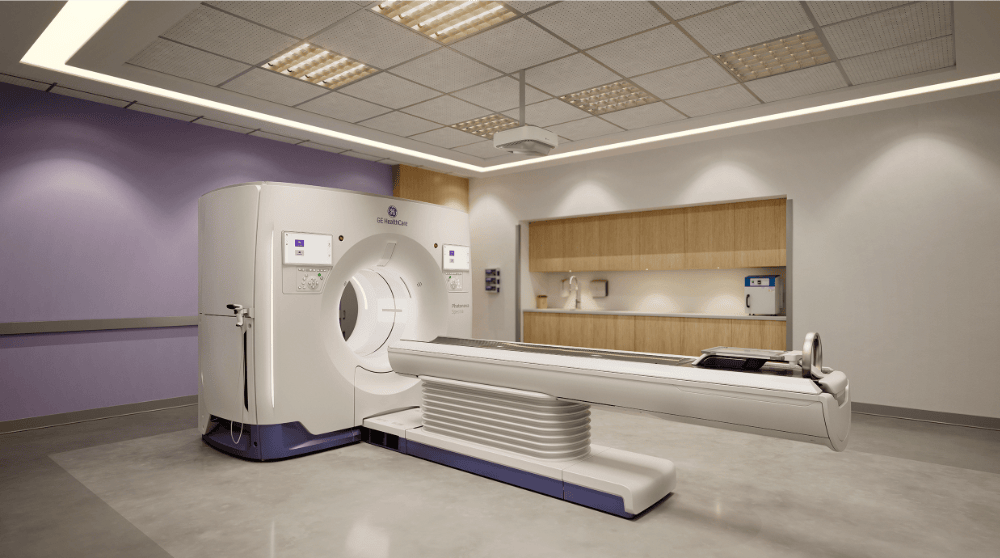
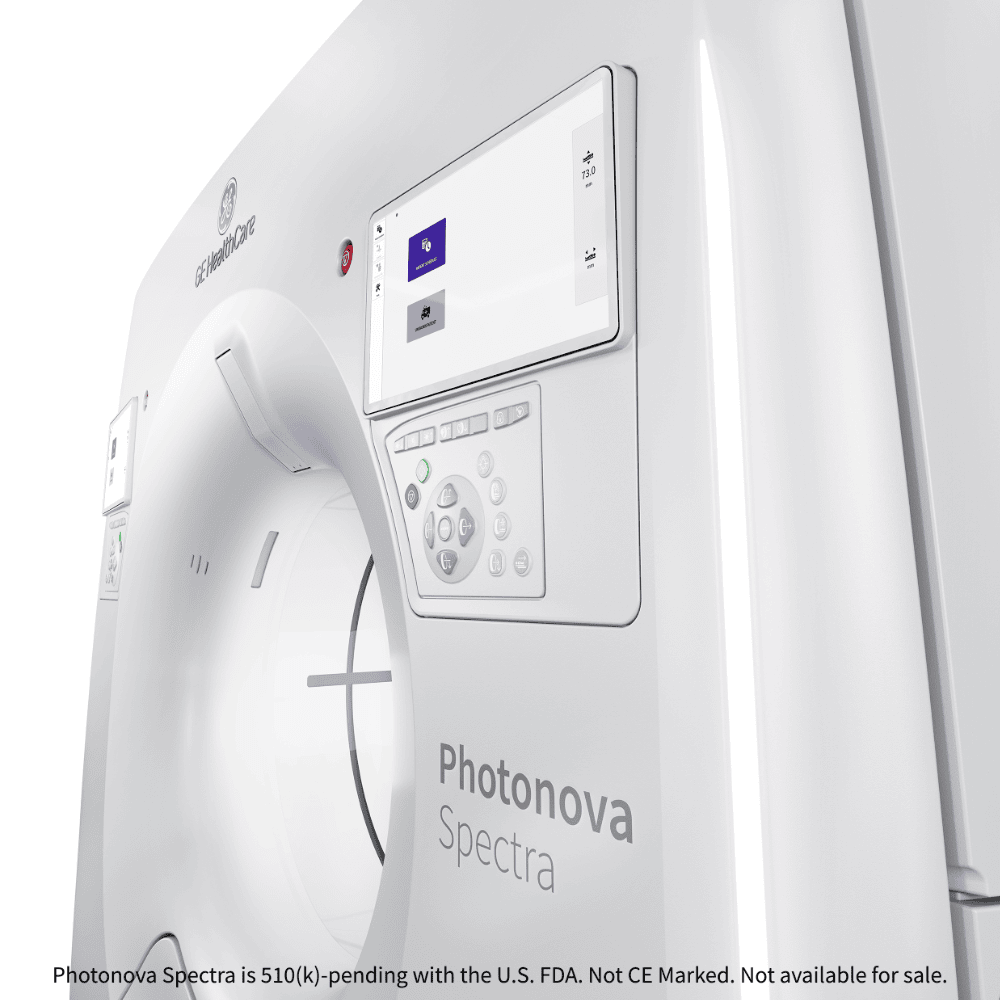
GE HealthCare unveils photon-counting CT with Deep Silicon detector
GE HealthCare has submitted a 510(k) application to the US FDA for Photonova Spectra, a photon-counting computed tomography (PCCT) system featuring the company’s proprietary Deep Silicon detector technology. The system offers 8-bin energy resolution and aims to deliver enhanced spectral and spatial imaging across multiple clinical specialties.
Direct photon detection enables enhanced tissue characterisation
Unlike conventional CT systems that convert X-rays into light before measurement, photon-counting CT directly counts individual X-ray photons and measures their energy levels. The Deep Silicon detector design uses the semiconductor’s structural consistency and purity to enable precise photon energy measurement, supporting improved contrast resolution and material characterisation capabilities.
“Photon counting CT is a fundamentally different approach to imaging. It can be thought of as particle physics in action,” said Dr Giuseppe Toia, Assistant Professor of Radiology at the University of Wisconsin School of Medicine and Public Health. “Being involved in developing and testing the Deep Silicon detector has allowed us to assess how the technology can be applied to address common issues such as improving spatial resolution and attaining accurate CT numbers.”
System design targets multiple clinical applications
The system’s clinical design goals span neurology, oncology, musculoskeletal imaging, thoracic imaging and cardiology. Technical specifications include wide detector coverage and 0.23-second rotation speed to support rapid acquisition and motion-free imaging. The system’s architecture aims to distinguish between materials including iodine, calcium and fat through enhanced energy separation capabilities.
Peter Arduini, President and Chief Executive Officer of GE HealthCare, stated: “Built to give healthcare teams the clarity and confidence they need, this system aims to redefine decision-making and care delivery – meeting today’s challenges and tomorrow’s possibilities.”
Accelerated computing integration supports data processing
Photonova Spectra incorporates NVIDIA’s accelerated computing technology to process the increased data volumes generated by photon-counting acquisition. According to GE HealthCare, the system harnesses up to 50 times more data than conventional CT systems when compared to the Revolution Apex Elite platform.
“By working on the technology’s development and pursuing opportunities to pair GE HealthCare’s Deep Silicon architecture with NVIDIA’s Blackwell platform in the future, we aim to unlock the full potential of spectral imaging – turning massive volumes of data into actionable insights,” said Kimberly Powell, Vice President of Healthcare at NVIDIA.
The system features universal full-fidelity scanning protocols designed to reduce exam-specific setup requirements whilst enabling automated reconstruction of ultra-high-resolution spectral images. The CT ONE operator environment includes automated features such as Auto Positioning to support workflow consistency.
Regulatory status: Photonova Spectra is 510(k) pending with the US FDA. It is not yet CE marked.
- For more information, visit: gehealthcare.com