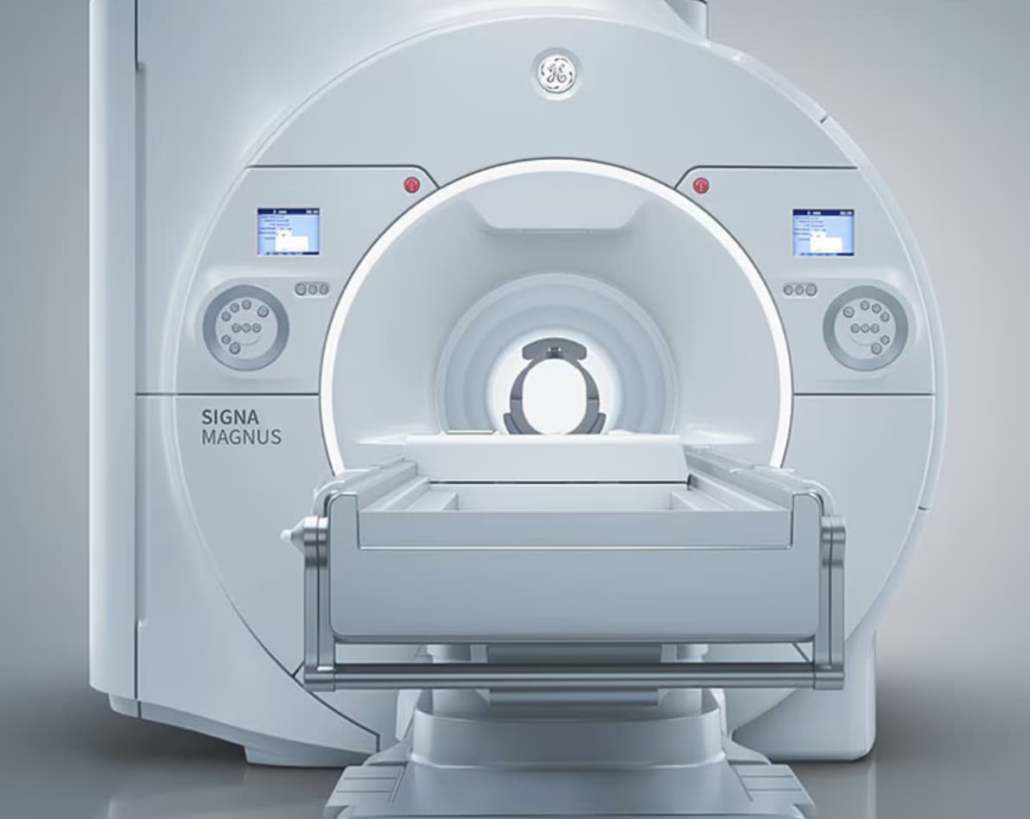
GE HealthCare unveils groundbreaking head-only MRI scanner
GE HealthCare has introduced SIGNA MAGNUS, a novel head-only magnetic resonance imaging (MRI) scanner designed to advance neuroscience research. The device, which is currently pending FDA 510(k) clearance (it is not yet CE marked), aims to overcome the performance limitations of conventional whole-body MR systems that have historically constrained neurological and psychiatric research.
With 43% of the global population affected by neurological disorders, there is a pressing need to expand the neurological clinical applications of MRI. SIGNA MAGNUS represents GE HealthCare’s most advanced 3.0T MR imaging device, specifically engineered for high-standard neurological and oncological research in head-only imaging.
Technical specifications and capabilities
SIGNA MAGNUS boasts superior gradient performance through its HyperG gradient technology, featuring 300 mT/m and 750 T/m/s. This enables the detection of previously unattainable fine details. The system’s innovative asymmetric gradient design allows for exceptional diffusion performance, achieving extremely high B-value diffusion with short echo times (TEs), which enhances the understanding of neural architecture.
Potential for research advancement
The device is designed to push the boundaries of advanced anatomical, diffusion, and functional techniques. It is augmented by GE HealthCare’s latest deep-learning algorithms, potentially uncovering new parameters and biomarkers for neurological research.
Kelly Londy, CEO of MR GE HealthCare, said: “With SIGNA MAGNUS, we are not just exploring the possibility of providing the tool; we are setting new benchmarks in medical research and future clinical patient care. This innovation underscores our commitment to R&D and our collaborations with academia, pushing the boundaries of what’s possible in MR imaging. The potential impact of SIGNA MAGNUS on patient outcomes and our understanding of the human brain is profound.”
Collaboration with leading research institutions
In March 2024, an investigational MAGNUS system was installed at Brigham and Women’s Hospital, a prominent research institution. The Brigham team will collaborate with GE HealthCare in conducting research on high-performance neuro MR.
Implications for future research and patient care
SIGNA MAGNUS represents a significant step forward in neuroimaging technology, potentially empowering neuroscientists, neurologists, neuroradiologists, and oncologists to transcend current barriers in diagnosis, understanding, and treatment of complex neurological and psychiatric diseases.
The system’s advanced capabilities may facilitate more in-depth exploration of brain microstructure, microvasculature, and function. This could lead to improved diagnostic accuracy and enhanced understanding of conditions such as Alzheimer’s disease and other neurological disorders that have remained challenging to diagnose and treat effectively.
Upgradeability and cost considerations
GE HealthCare has designed SIGNA MAGNUS with upgradeability in mind. Many of their existing 3.0T systems can be upgraded to SIGNA MAGNUS, potentially reducing capital costs for healthcare institutions and research facilities.
- For more information, visit: GE Healthcare Signa Magnus MRI