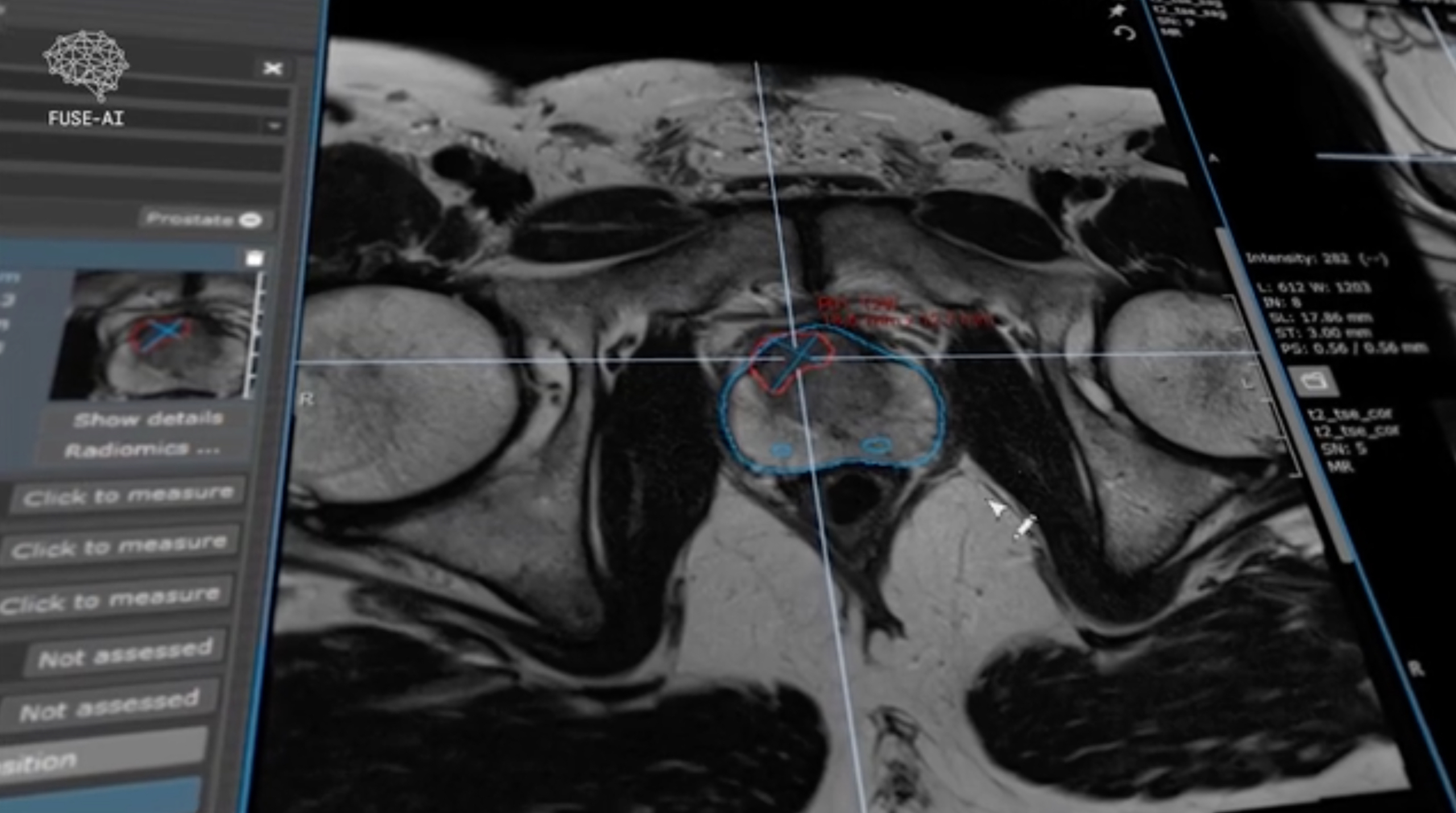
FUSE-AI’s AI algorithm for radiology of the prostate receives MDR certification
German company FUSE-AI, a developer of artificial intelligence for radiology, has received EU Medical Device Regulation (MDR) 2017/745 IIa certification for its AI software “Prostate.Carcinoma.ai”.
Prostate.Carcinoma.ai significantly aids radiologists by automatically segmenting the prostate gland in MRI scans and independently identifying pathological changes, providing a 30% time saving per patient. This efficiency translates into considerable financial benefits for radiology centres and departments. The software’s integration into existing clinical software systems ensures user-friendliness and preserves radiologists’ familiarity with their work environment.
“This AI software is a testament to the interdisciplinary collaboration among scientists, AI developers, and radiologists. Receiving certification is a pivotal step, transitioning preliminary agreements into binding contracts and fully leveraging the software’s capabilities in clinical settings,” said Matthias Steffen, Founder and CEO of FUSE-AI.
For more information, visit: https://fuse-ai.de/
Prostate.Carcinoma.ai is the spearhead for a suite of AI-powered diagnostic assistance software for various medical indications, positioning FUSE-AI for global clinical presence and making the software an indispensable tool in medical diagnostics.
Digital issue: Please click here for more information