First pig-to-human liver transplant reported
A landmark case published in the Journal of Hepatology describes the world’s first auxiliary liver xenotransplant from a genetically engineered pig to a living human recipient, with the patient surviving 171 days. The procedure demonstrates that genetically modified porcine livers can support essential hepatic functions in humans, whilst highlighting significant complications that must be addressed before wider clinical application.
The successful transplantation of a genetically modified pig liver into a 71-year-old man represents a significant milestone in addressing the global organ shortage crisis. Published on 9 October 2025 in the Journal of Hepatology, the case report provides proof-of-concept that xenotransplantation could offer a viable alternative to human donor organs, though substantial challenges remain.
A landmark study in the Journal of Hepatology reports the world’s first auxiliary liver xenotransplant from a genetically engineered pig to a living human recipient. © Journal of Hepatology / Zhang et al.
Global organ shortage drives xenotransplantation research
According to the World Health Organisation, thousands of patients die annually whilst awaiting organ transplants due to limited organ availability. In China, where the procedure took place, hundreds of thousands of people experience liver failure each year, yet only approximately 6,000 liver transplants were performed in 2022. This disparity between demand and supply has intensified research efforts into xenotransplantation as a potential solution.
Case details and surgical approach
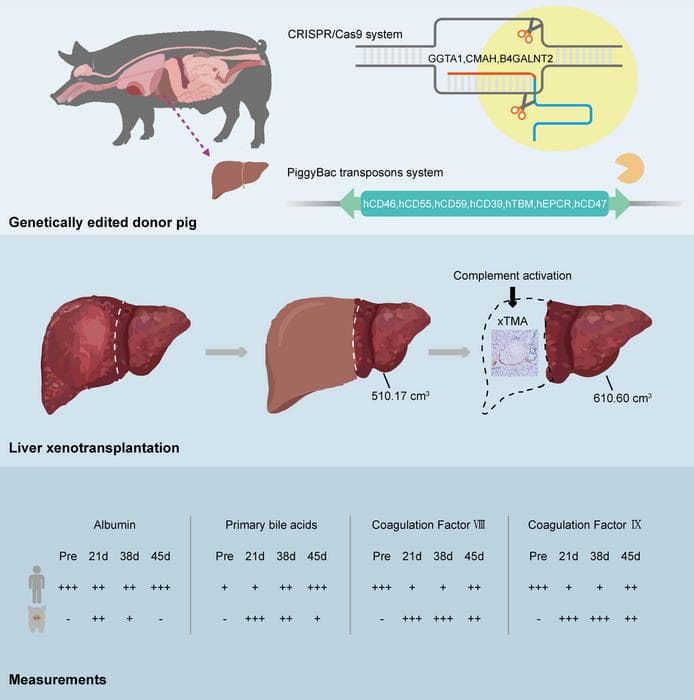
The recipient was a 71-year-old man with hepatitis B-related cirrhosis and hepatocellular carcinoma who was ineligible for surgical resection or conventional human liver transplantation. Surgeons implanted an auxiliary graft from a Diannan miniature pig that had undergone 10 genetic modifications, including xenoantigen knockouts and human transgenes designed to enhance immune and coagulation compatibility.
Initial graft function and complications
During the first month post-surgery, the xenograft demonstrated effective function, producing bile and synthesising coagulation factors without evidence of hyperacute or acute rejection. However, on day 38, surgeons removed the graft following the development of xenotransplantation-associated thrombotic microangiopathy (xTMA), a serious complication involving complement activation and endothelial injury.
Treatment with the complement inhibitor eculizumab combined with plasma exchange successfully resolved the xTMA. Despite this intervention, the patient subsequently experienced repeated episodes of upper gastrointestinal haemorrhage and died on day 171.
Clinical significance and future implications
“This case proves that a genetically engineered pig liver can function in a human for an extended period,” explained lead investigator Beicheng Sun, MD, PhD, from the Department of Hepatobiliary Surgery at the First Affiliated Hospital of Anhui Medical University. “It is a pivotal step forward, demonstrating both the promise and the remaining hurdles, particularly regarding coagulation dysregulation and immune complications, that must be overcome.”
The case demonstrates that whilst genetically modified porcine livers can engraft and deliver key hepatic functions, significant biological challenges persist. The development of xTMA and subsequent complications underscore the need for improved strategies to manage coagulation disorders and immune responses in xenotransplantation recipients.
A new era for transplant hepatology
In an accompanying editorial, Heiner Wedemeyer, MD, Co-Editor of the Journal of Hepatology, described the report as “a landmark in hepatology.” He noted that xenotransplantation may open new therapeutic avenues for patients with acute liver failure, acute-on-chronic liver failure, and hepatocellular carcinoma, declaring that “a new era of transplant hepatology has started.”
However, the editorial also emphasised the biological and ethical challenges that must be addressed before xenotransplantation can be translated into wider clinical use. These include optimising genetic modifications to prevent thrombotic complications, refining immunosuppression protocols, and establishing appropriate patient selection criteria.
Research implications
The successful engraftment and function of the genetically modified porcine liver, even for a limited period, provides valuable data for future xenotransplantation attempts. The case highlights specific areas requiring further research, particularly the prevention and management of xTMA and coagulation abnormalities that appear to be critical barriers to long-term graft survival.
Vlad Ratziu, MD, PhD, Editor in Chief of the Journal of Hepatology, stated: “The publication of this case reaffirms the Journal of Hepatology as the world’s leading liver journal. We are committed to presenting cutting-edge translational discoveries that redefine what is possible in hepatology.”
As xenotransplantation research advances, this case will serve as an important reference point for understanding both the potential and limitations of cross-species organ transplantation in addressing the critical shortage of human donor organs.
Reference
Zhang, W., Xu, Q., Xu, K., Jiang, R., Wang, S., Zheng, M., Liu, N., Jiao, D., Wang, Z., Ge, J., Lu, X., Li, G., Huang, F., Liu, L., Yin, Y., Liu, Y., Guo, J., Liu, K., Wei, H.-J., & Sun, B. (2025). Genetically modified porcine-to-human liver xenotransplantation. Journal of Hepatology, 84(3). https://doi.org/10.1016/j.jhep.2025.08.044

