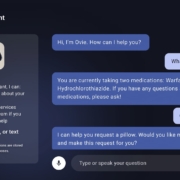
Oneview launches GenAI care assistant Ovie to reduce nursing workload
Oneview Healthcare has developed a GenAI-powered care assistant called Ovie that aims to provide 24/7 support for patients while alleviating nursing burden. The voice-activated system integrates with hospital electronic health records to deliver personalised responses to patient queries and requests.