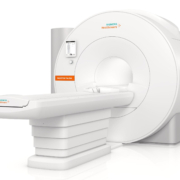
Canon Medical launches Vantage Fortian 1.5T MRI with AI to accelerate workflows
Canon Medical recently unveiled the Vantage Fortian – a new, advanced productivity, open bore 1.5T MRI system. The new system features innovative workflow solutions, image enhancement and accelerated scan technology, which together, can contribute to reducing the time required for MRI procedures.