Large observational study reports 50% of hospitalised Covid-19 patients develop a complication
Researchers warn complications may cause a substantial strain on health and social care in the coming years
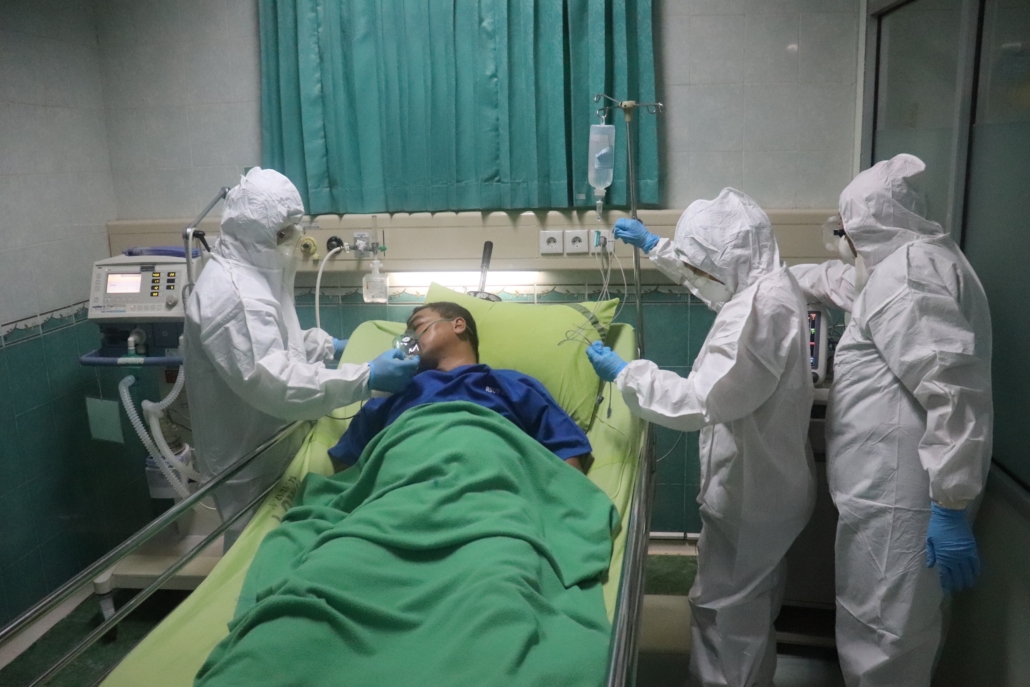
An observational study of more than 70,000 people in 302 UK hospitals finds that one in two people hospitalised with COVID-19 developed at least one complication. The new study, published in The Lancet [1], is the first to systematically assess a range of in-hospital complications, and their associations with age, sex and ethnicity, and their outcomes for the patients.
The authors say these complications are likely to have important short- and long-term impacts for patients, healthcare utilisation, healthcare system preparedness, and society amidst the ongoing COVID-19 pandemic. They also note that these complications are different to long COVID symptoms in patients with COVID-19 who were not hospitalised.
Risk to younger healthy adults
The authors say that complications in patients admitted to hospital with COVID-19 are high, even in young, previously healthy individuals – with 27% of 19-29 year olds and 37% of 30-39 year olds experiencing a complication. They also note that acute complications are associated with reduced ability to self-care at discharge – with 13% of 19-29 year olds and 17% of 30-39 year olds unable to look after themselves once discharged from hospital.
The study looked at cases between 17 January and 4 August 2020 before vaccines were widely available, and new variants of the virus had not arisen. However, the authors note that their findings remain relevant in dispelling suggestions that COVID-19 presents no risk to younger healthy adults, many of whom remain unvaccinated.
The authors warn that policymakers must consider the risk of complications for those who survive COVID-19, not just mortality, when making decisions around easing restrictions. The authors predict that COVID-19 complications are likely to cause significant challenges for individuals and for the health and social care system in the coming years. Policy makers and health-care planners should anticipate that large amounts of health and social care resources will be required to support those who survive COVID-19.
Chief Investigator and joint senior author of the study, Professor Calum Semple, University of Liverpool, UK, said: “This work contradicts current narratives that COVID-19 is only dangerous in people with existing comorbidities and the elderly. Dispelling and contributing to the scientific debate around such narratives has become increasingly important. Disease severity at admission is a predictor of complications even in younger adults, so prevention of complications requires a primary prevention strategy, meaning vaccination.”
Kidney, heart and lungs
Commenting on the research, joint senior author Professor Ewen Harrison from the University of Edinburgh, UK, said: “Patients in hospital with COVID-19 frequently had complications of the disease, even those in younger age groups and without pre-existing health conditions. These complications could affect any organ, but particularly the kidney, heart and lungs. Those with complications had poorer health on discharge from hospital, and some will have long-term consequences. We now have a more detailed understanding of COVID-19 and the risks posed, even to younger otherwise healthy people.”
He added: “Our review highlights some insightful patterns and trends that can inform healthcare systems and policy maker responses to the impacts of COVID-19. Our results can also inform public health messaging on the risk COVID-19 poses to younger otherwise healthy people at a population level, particularly in terms of the importance of vaccination for this group.”
Previous research on the impact of COVID-19 on patients has focussed on the numbers of deaths or on outcomes related to one specific organ system or health condition.
Data collection
The new study assessed in-hospital complications in adults aged 19 years or over with confirmed or highly suspected SARS-CoV-2 infection leading to COVID-19 disease. Data were collected by nurses and medical students, and included the participants’ age, sex at birth, health measures when hospitalised, and comorbidities (such as asthma, chronic cardiac disease, chronic haematological disease, chronic kidney disease, chronic neurological disease, chronic pulmonary disease, HIV/AIDS, cancer, liver disease, obesity, rheumatological disorders, and smoking).
In addition, they collected data on the respiratory, neurological, cardiovascular, renal, gastrointestinal and systemic complications participants experienced while in hospital [2]. Complications were assessed at multiple timepoints until discharge or, if the patient was not discharged, 28 days after hospitalisation. The study also investigated the ability of patients to look after themselves when discharged from hospital.
80,388 patients were included in the study, but 7,191 were excluded due to duplicated medical records, as they were not eligible for the study, or because no data was collected on the compilations they experienced while in hospital.
Of the remaining 73,197 patients, 56% were men, 81% had an underlying health condition, 74% were of white ethnicity, and the average age of the cohort was 71 years. Almost one in three participants (32%, 23,092 of 73,197) in the study died.
Most common complications
Overall, complications occurred in 50% of all participants, including in 44% (21,784 of 50,105) of participants who survived.
The most common complications were renal (affecting almost one in four people, 24%, 17,752), respiratory (affecting around one in five people, 18%, 13,486), and systemic (affecting one in six, 16%, 11,895). However, cardiovascular complications were reported in around one in eight participants (12%, 8,973), and neurological (less than one in 20, 4%, 3,115), and gastrointestinal or liver (less than 11%, 7,901) complications were also reported. Specifically, acute kidney injury, probable acute respiratory distress syndrome, liver injury, anaemia, and cardiac arrhythmia were the most common complications.
The incidence of complications rose with increasing age, occurring in 39% (3,596 of 9,249) of 19-49 year olds, compared to 51% (32,771 of 63,948) of people aged 50 and older. Going up the age ranges, 27% of 19-29 year olds hospitalised with COVID-19 developed a complication, 37% of 30-39 year olds, 43% of 40-49 year olds, 49% of 50-59 year olds, 54% of 60-69 year olds, 52% of 70-79 year olds, 51% of 80-89 year olds, and 50% of people aged 90 or over (see Table 1).
Complications were more common in men compared with females, with males aged older than 60 years the most likely group to have at least one complication (women aged under 60 years: 37% [2,814 of 7,689] and men 49% [5,179 of 10,609]; women aged 60 years and over: 48% [11,707 of 24,288] and men 55% [16,579 of 30,416]).
People of white, South Asian, and East Asian ethnicities had similar rates of complications, but rates were highest in Black people (58% [1,433 of 2,480] in Black patients vs 49% [26,431 of 53,780] in white patients).
Self-care compromised
Following hospitalisation, 27% (13,309 of 50,105) of patients were less able to look after themselves than before COVID-19, and this was more common with older age, being male, and in people who received critical care. The association between having a complication and worse ability for self-care remained irrespective of age, sex, socioeconomic status, and which hospital someone received treatment in. Neurological complications were associated with the biggest impact on ability for self-care.
Strain on health and social care resources
Based on these rates, the authors say that policymakers and healthcare planners should anticipate that large amounts of health and social care resources will be required to support those who survive COVID-19. This includes adequate provision of staffing and equipment – for example, provision of follow-up clinics for those who have sustained in-hospital complications such as acute kidney injury or respiratory tract infection.
Dr Thomas Drake, co-author from the University of Edinburgh, UK, said: “Our research looked at a wide range of complications, and found that short-term damage to several organs is extremely common in those treated in hospital for COVID-19. These complications were common in all age groups, not just in older people or those with pre-existing health conditions. People who have complications will often need expert care and extra help to recover from their initial hospital admission. Our study shows it is important to consider not just death from COVID-19, but other complications as well. This should provide policy makers with data to help them make decisions about tackling the pandemic and planning for the future. We are still studying the participants in our study to understand what the long-term effects of COVID-19 on their health. The results from these ongoing studies will be particularly useful, as we found many people who survive COVID-19 and develop complications are from economically active age groups.”
Aya Riad, joint co-author from the University of Edinburgh, UK, said: “It is important that with the high risk of complications and the impact these have on people, that complications of COVID-19, not just death, are considered when making decisions on how best to tackle the pandemic. Just focussing on death from COVID-19 is likely to underestimate the true impact, particularly in younger people who are more likely to survive severe COVID-19.”
The authors note that around 85% of participants had a positive SARS-CoV-2 RT-PCR test, and patients who did not have a positive test recorded similar or slightly lower rates of in-hospital complications.
Study limitations
They also note some limitations, including that the data does not provide a long-term picture, and that the timings of complications and patients quality of life were not studied. In addition, the complications in the study were predefined and not specific to COVID-19, so may underestimate some areas as these were added later. In addition, as it was inappropriate to subject patients to numerous tests, patients did not undergo additional tests for complications, and the authors say that the true burden of complications is likely to be higher.
References
[2] Data were collected on organ-specific complications including complex respiratory (bacterial pneumonia, acute respiratory distress syndrome [ARDS], empyema, pneumothorax, and pleural effusion), neurological (meningitis, encephalitis, seizure, and stroke), cardiovascular (thromboembolism, heart failure, myocarditis, endocarditis, arrhythmia, cardiomyopathy, myocardial ischaemia, and cardiac arrest), acute kidney injury, gastrointestinal (acute liver injury, pancreatitis, and gastrointestinal haemorrhage), and other systemic complications (coagulopathy, disseminated intravascular coagulation, anaemia, and bloodstream infection).