Catalyst OrthoScience receives FDA clearance for novel shoulder fracture system
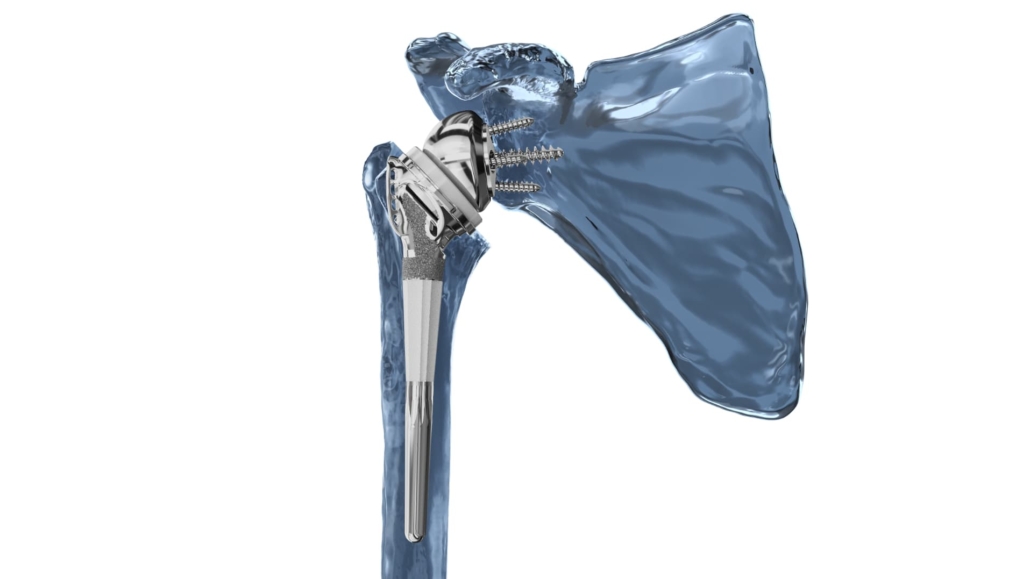
Catalyst OrthoScience, a medical technology company specialising in shoulder arthroplasty, has announced FDA 510(k) clearance for its Catalyst Fracture Shoulder System. The system, designed for reverse shoulder arthroplasty in cases of proximal humeral fractures (PHFs), aims to tackle the complexities associated with tuberosity fixation and healing.
Key features of the system
The Catalyst Fracture System incorporates several design elements to address the specific challenges of PHF treatment:
- A tuberosity-specific fossa to maximise contact and healing with a porous surface
- Patented tuberosity retention rails to restrict post-operative motion
- Uncemented, press-fit diaphyseal fixation for rotational stability, with cemented options available
- Compatibility with various glenosphere and baseplate configurations
Clinical perspective
Dr Steven Goldberg, chief medical officer of Catalyst, highlighted the increasing use of reverse shoulder arthroplasty for PHF treatment. He noted: “Catalyst has engineered a solution that specifically addresses the complexities of PHFs in an efficient and novel way.”
Dr John A. Brown, a co-designer of the system, emphasised its potential benefits for surgeons operating in both hospitals and ambulatory surgery centres, saying that the system is “specifically designed to enhance tuberosity fixation and healing, which is consistently shown to produce better outcomes”.
Initial clinical experience
One of the first procedures using the new system was performed by Dr Matthew D. Budge, who reported: “The unique design of the proximal body greatly simplified the usual difficulties encountered with tuberosity management. In addition, the ability to get excellent diaphyseal fixation of the implant without cement or screws greatly improved the efficiency of the case.”
Future plans
Catalyst intends to conduct a limited user release to gather additional data on the system’s performance before a broader commercial release planned for 2025.
- For more information, visit: catalystortho.com