Breakthrough magnetic capsule robot integrates diagnosis and treatment for gastrointestinal diseases
Researchers at the University of Macau and University of Hong Kong have developed an innovative multichamber capsule robot that selectively samples bodily fluids and delivers targeted medications via magnetic field control. This breakthrough addresses fundamental limitations in current capsule endoscopy by enabling integrated diagnosis and treatment through single oral administration.
The multichamber magnetic capsule robot (macabot) features four independent chambers equipped with magnetic valves demonstrating directional stiffness properties. Each valve comprises a four-bar parallelogram compliant mechanism embedded with a tiny permanent magnet (Φ1.5 mm × 2 mm). The device measures Φ9.8 mm × 20 mm, fitting within standard capsule dimensions.
The innovation enables selective opening of individual chambers via directional gradient magnetic fields, active navigation through rotating magnetic field locomotion, liquid sampling at multiple sites without cross-contamination, and precise, on-demand drug release at targeted locations. Unlike passive capsule technologies relying on intestinal peristalsis, the macabot utilises programmable magnetic field actuation for precise spatial control.
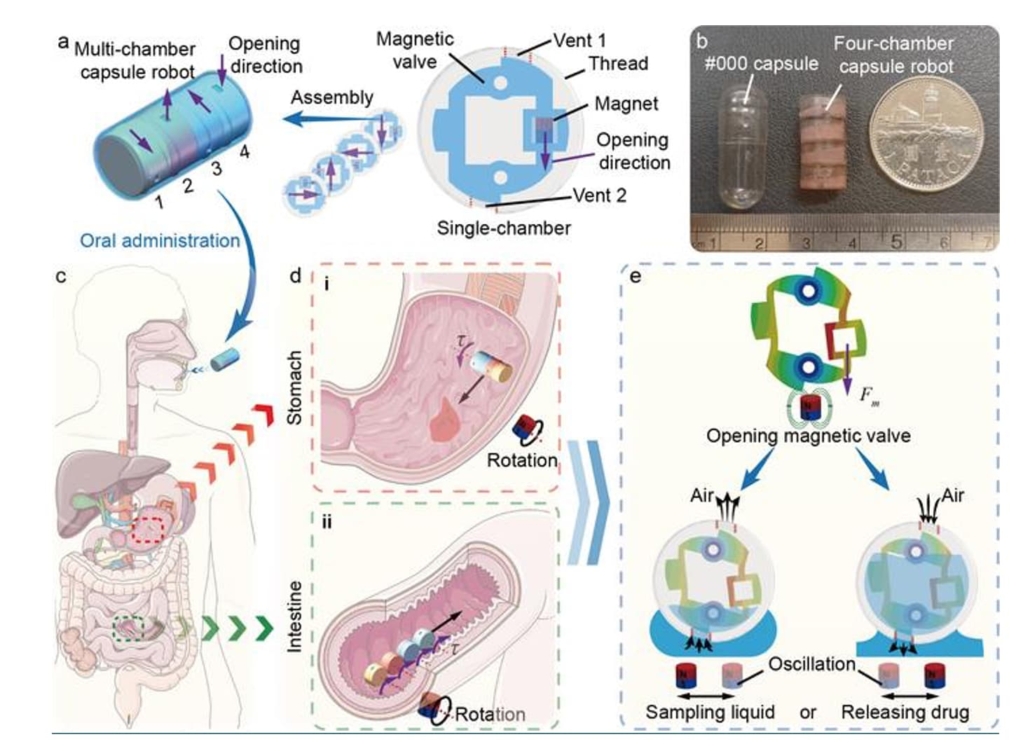
Illustration of the mechanical design and workflow of the macabot. (a) Schematic of a multichamber capsule robot with four single-chamber capsules with decoupled opening directions. (b) Photograph of the fabricated macabot. (c) Schematic of macabot navigating in the digestive tract by oral administration. (d) Schematic of macabot rolling navigation in (i) the stomach and (ii) the intestine. (e) Schematic of the macabot sampling liquid and releasing the drug by opening the magnetic valve.
Addressing critical clinical needs
Gastrointestinal diseases account for over 25% of all cancer cases and more than 33% of cancer-related deaths worldwide. Current treatment approaches face substantial limitations: drug therapies suffer from poor targeting, leading to excessive dosing and adverse effects; traditional capsule endoscopy creates diagnostic blind spots through passive movement; existing magnetic capsules cannot perform simultaneous diagnosis and treatment.
The macabot integrates biopsy and medication capabilities, allowing physicians to sample bodily fluids at suspected lesion sites and immediately deliver targeted therapy during a single procedure—potentially transforming clinical workflows for gastrointestinal disease management.
Engineering excellence through compliant mechanisms
The selective opening mechanism represents the core innovation. Each chamber’s magnetic valve exhibits low stiffness in its designated triggering direction whilst remaining rigid under perpendicular or opposing forces. When gradient magnetic field force aligns with the valve’s opening direction, the compliant mechanism deforms elastically, exposing two vents for liquid sampling or drug release.
This decoupled activation mechanism allows capsule rolling through the digestive tract without accidental chamber opening whilst enabling precise control during sampling or release operations. The magnetic torque generated during rolling locomotion transmits to the capsule shell without triggering valve deformation, demonstrating sufficient rotational stiffness for reliable navigation.
Comprehensive experimental validation
Selective opening performance
Simulation studies confirmed magnetic valves deform exclusively when magnetic force aligns with their opening direction. Experimental testing with various flexure hinge parameters demonstrated tunable activation thresholds. Sealing tests revealed less than 2% liquid loss during intensive 500 rpm rotation for five minutes, confirming reliability exceeding normal physiological activity.
Navigation capabilities
The macabot achieved rolling velocities up to 0.38 m/s under 10 mT rotating magnetic fields. Stable movement occurred at frequencies below 2 Hz with minimal velocity variation. The device successfully navigated complex trajectories in integrated four-chamber mode and demonstrated parallel navigation as split individual modules through 20 mm diameter tubing. pH-sensitive adhesives enable automatic splitting when transitioning from gastric to intestinal environments, facilitating navigation through narrower passages.
Liquid sampling results
Experimental studies demonstrated successful fluid sampling from liquids with varying densities. Sampled volumes increased proportionally with liquid density, approaching approximately 50% chamber capacity. Linear oscillation of the external magnet significantly enhanced fluid flow. Multi-site sampling experiments across four locations with different pH values (6-9) confirmed no cross-contamination—pH measurements from sampled liquids matched original solution values, validating separate chamber integrity.
Targeted drug delivery
Release experiments demonstrated precise drug positioning with average release areas of 19.18 mm × 8.85 mm. The system demonstrated robust reproducibility across three release cycles with minimal variation. Mucus clearance capability significantly enhanced drug bioavailability by enabling release beneath the mucosal barrier. Sequential selective release from multiple chambers validated combination therapy potential.
Advanced sustained-release application
Beyond instantaneous drug delivery, the macabot enables in situ hydrogel patch formation for sustained therapeutic release—valuable for chronic conditions like inflammatory bowel disease. By storing sodium alginate solution and calcium chloride solution in separate chambers, the system generates shape-adaptive hydrogel patches directly at lesion sites. Fluorescein isothiocyanate release studies confirmed sustained drug delivery over multiple hours.
Ex vivo clinical validation
Researchers validated macabot functionality in ex vivo porcine stomach tissue under ultrasound imaging guidance. A dual-chamber configuration successfully performed liquid sampling at one site followed by drug release at a different location. Ultrasound displacement measurements matched bright-field observations, confirming real-time visualisation feasibility using existing clinical imaging infrastructure.
The 3.8 mN force applied to tissue (74 Pa pressure across 51.29 mm² contact area) remained significantly below standard gastroscopy inflation pressure (1.33 kPa), ensuring patient safety. These results validate the potential for clinical translation using established medical imaging systems.
Future development pathways
Several avenues exist for enhanced functionality. Pre-deformation techniques could enable eight or more chambers with increased angular resolution between triggering directions, though requiring higher magnetic field gradients. Anisotropic magnetisation approaches could enable six degree-of-freedom motion with walking and swinging locomotion modes complementing rolling.
Adaptation for bile and pancreatic ducts requires addressing size constraints (potentially 7.8 mm diameter) whilst maintaining fluid interaction through smaller vents. Essential next steps include in vivo studies assessing performance under dynamic gastrointestinal conditions, including intestinal peristalsis effects on operational accuracy and stability.
Clinical impact and applications
This research addresses fundamental capsule endoscopy limitations by enabling integrated diagnosis and treatment through one-time oral intake, multiple-site sampling without cross-contamination, on-demand combination therapies, and sustained-release patch formation at lesion sites.
The technology shows particular promise for diseases with multiple lesions, such as Crohn’s disease, where targeted multi-site delivery during single administration offers substantial advantages over conventional oral medications. Patients benefit from reduced physical and psychological burdens compared to separate diagnostic and therapeutic procedures.
Conclusion
The multichamber magnetic capsule robot represents significant progress towards integrated diagnostic and therapeutic platforms for gastrointestinal diseases. By enabling selective chamber activation through elegant compliant mechanism design, the technology overcomes the fundamental conflict between manufacturing simplicity and functional enhancement characterising existing capsule robots.
Successful validation through comprehensive experimental studies demonstrates approach feasibility. With continued development including in vivo testing and potential clinical trials, the macabot could provide a transformative tool for minimally invasive, patient-friendly management of gastrointestinal diseases affecting millions worldwide.
The findings, published in National Science Review, represent the culmination of extensive simulation, in vitro, and ex vivo validation studies demonstrating the feasibility and potential of this integrated diagnostic and therapeutic platform.
Reference
Zehao Wu, Xianli Wang, Yang Lu, Qingsong Xu, Multichamber magnetic capsule robot for selective liquid sampling and drug delivery, National Science Review, 2025;, nwaf400, https://doi.org/10.1093/nsr/nwaf400

