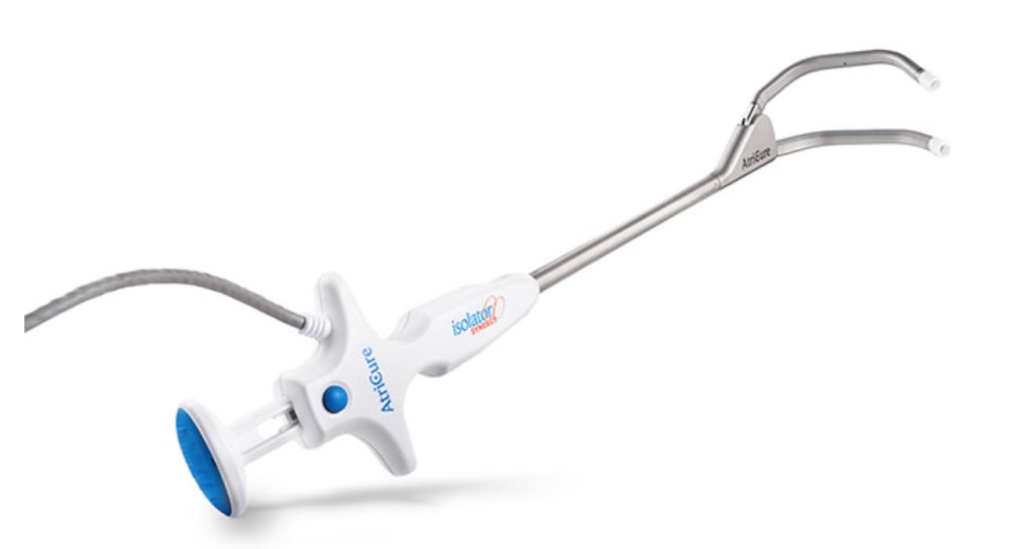
AtriCure’s EnCompass Clamp receives CE mark approval
AtriCure has announced regulatory approval for the sale of its EnCompass® Clamp in CE-marked countries within the European Union. This development follows the device’s FDA 510(K) clearance and subsequent launch in the United States in 2022.
Device features and functionality
The EnCompass Clamp is designed to simplify and expedite cardiac ablation procedures in open-chest surgeries. It facilitates comprehensive epicardial ablation of the left atrium, potentially reducing procedure time to a matter of minutes. The device incorporates features from AtriCure’s existing Synergy™ Clamp family, including parallel closure, uniform pressure, and customised power delivery using Synergy radiofrequency (RF) technology.
A notable innovation in the EnCompass Clamp is its magnetic guide system, which allows for more efficient placement with minimal tissue dissection. The clamp’s design accommodates various cardiac anatomies, supporting surgical ablation in procedures where the atrium would typically remain closed, such as coronary artery bypass grafting (CABG) and aortic valve replacement (AVR).
Clinical implications
AtriCure estimates that approximately 400,000 cardiac surgeries are performed annually in the European Union, suggesting a significant potential market for the EnCompass Clamp.
Michael Carrel, President and CEO of AtriCure, commented: “We have seen this product have a positive impact in the United States over the last two years by advancing treatment concomitant to cardiac surgery. We are excited to offer this safe, innovative, and effective therapy to patients and our physician partners in Europe.”
- For more information, visit: atricure.com