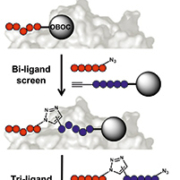
Protein catalysed capture agents for molecular imaging
There is a growing need for affinity agents for targeted molecular imaging of disease biomarkers. Protein catalysed capture agents (PCC agents) are assembled through target-guided in situ click chemistry and display low nanomolar affinity and specificity. The iterative design process, architecture, and composition of the resulting multi-ligands make this a promising technology for imaging agent development.
by Dr Steven W. Millward, Dr Heather D. Agnew, Dr Suresh Pitram, Dr Bert T. Lai, Dr Rosemary D. Rohde and Dr Norman Hardman
Personalized medicine is increasingly becoming a major component of cancer treatment and management. Many of the advances in personalized medicine have come from high throughput genomic sequencing and multiplexed proteomic analysis of tumour and tissue specimens. While these technologies have dramatically increased our understanding of cancer and cancer heterogeneity at the molecular level, translation of this knowledge into clinical imaging agents to non-invasively characterize tumour phenotypes in vivo has lagged behind. While small molecules (e.g. FDG) have proven very useful for clinical measurement of metabolic activity, molecular imaging of cell surface and secreted biomarkers overexpressed in cancer (e.g. IGFR, EGFR, etc.) is largely confined to the preclinical and early clinical trial setting.
While groundbreaking work to determine the biological and therapeutic roles of these proteins has produced a wealth of high affinity antibodies, antibody fragments, and peptides, attempts to translate them into radiotracers for molecular imaging have produced mixed results. Antibody-based imaging agents suffer from three disadvantages in this setting:
1) Their long serum half-lives, while advantageous for therapeutic applications, result in high background signal in perfused tissue and lengthen the time between agent administration and image acquisition.
2) Antibody-based radiotracers often show accumulation and metabolic processing in the liver, increasing nonspecific signal and potentially obscuring critical tumour-specific signal in the abdominal cavity.
3) Antibodies are biologicals, and as such their high production costs and regulatory burdens are significant economic barriers to commercialization and clinical translation. Linear peptides derived from phage display represent a second class of potential imaging agents, however their rapid degradation in vivo typically results in nonspecific background signal and poor tumour uptake. An ideal targeted molecular imaging agent would combine the affinity and specificity of antibodies with the high bio-stability, tumour uptake, and clearance of small molecules.
PCC agent design
Protein catalysed capture (PCC) agent technology represents a novel approach to rapidly design ligands with high affinity and specificity that can be potentially adapted to a variety of research and in vivo clinical applications including molecular imaging. This technology was built upon the observation by K. Barry Sharpless and co-workers at The Scripps Research Institute that the azide-alkyne cycloaddition (a