Automation and integration of LC-MS/MS services into the clinical laboratory workflow
Despite significant inherent advantages of liquid chromatography-tandem mass spectrometry (LC-MS/MS) over immunoassay techniques in clinical laboratory applications, its adoption into routine practice has been slower than might have been expected. The barriers to more widespread uptake are a function of issues in the laboratory workflow. This article analyses those issues and discusses how they can be overcome by improved automation and integration with the laboratory information management system, drawing on examples from the North West London Pathology (NWLP) clinical laboratories at Imperial College Healthcare NHS Trust.
by Dr Emma L. Williams
Introduction
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) has seen over two decades of use in specialist clinical laboratories in the UK, offering a number of significant advantages over immunoassay techniques. These advantages include increased specificity, sensitivity and accuracy, as well as the detection of multiple analytes within a single assay. There is no need for an antibody for analyte detection and the method is not susceptible to the antibody-based interferences that plague immunoassays [1]. LC-MS/MS is suitable for multiple sample matrices and avoids the need for radioactive tracers. LC-MS/MS assays also have a wider dynamic measurement range and have improved between-method bias when compared to immunoassays.
LC-MS/MS initially played a role in specialist clinical laboratories in areas such as newborn screening, inborn errors of metabolism, toxicology and in immunosuppressant and therapeutic drug monitoring. More recently LC-MS/MS has established a role in diagnostic endocrinology, with the first appearance of LC/MS-MS for the measurement of vitamin D in the international vitamin D external quality assurance scheme (DEQAS) in 2005. There are now over 150 labs registered in this scheme using LC/MS-MS for the measurement of vitamin D. However, automated immunoassay still dominates and represents 69% of participants registered in the DEQAS scheme. Why has there not been more widespread adoption?
A number of issues have inhibited wider adoption and routine use of LC/MS-MS in the clinical laboratory. First among these is the use of labour-intensive manual workflows, which result in lower throughput, decreased productivity and longer turnaround time. Furthermore, a high level of technical expertise is needed, not only for method development, but also for troubleshooting assay and equipment failures. In addition to the high initial capital costs of purchasing the equipment, ongoing personnel costs are higher because of the need for more technically competent staff. With a clear understanding of where the bottlenecks in the process arise, these barriers can be overcome.
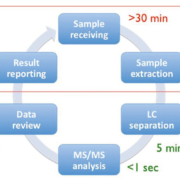
Figure 1 depicts the six main steps of a typical LC/MS-MS workflow, from sample receipt and extraction, separation in the LC, MS/MS analysis, data review and reporting of the results [2]. Of these steps it is the pre- and post-analytical stages that are the most time consuming and therefore if there is a focus on streamlining these, maximum benefit can be achieved. A number of steps can be taken to streamline the workflow, and these come under three broad headings of reduced manual processes, increased throughput and improved integration. Dependence on manual processes can be reduced by the automation of liquid handling and extraction, use of barcode reading for worklist generation and implementation of automated data analysis. Throughput can be increased with strategic column and sample management and by analyte multiplexing. Integration can be improved by bi-directional interfacing of the LC/MS-MS system to the laboratory information management system (LIMS) allowing automatic worklist upload and results download. These three strategic areas will be discussed in more detail below.
Reduced manual processes
Unlike the case with immunoassay, samples for LC-MS/MS usually require extraction prior to analysis. Historically this extraction step utilized liquid–liquid extraction or protein precipitation, these being carried out after the addition of internal standard to the calibrators, quality controls and patient samples. All of these steps involved manual pipetting and were very slow and time consuming. Use of an automated liquid-handling platform for the pipetting of samples and addition of internal standard allows some of the steps of liquid–liquid extraction and protein-precipitation methods to be automated. These liquid-handling platforms are available from a number of suppliers including Hamilton and Tecan.
With the advent of 96-well plate technology it became possible to carry out fully automated off-line solid phase extraction (SPE) using platforms such as the Freedom Evo (Tecan) and the Biomek NX (Beckman Coulter). More recently, supported liquid extraction (SLE), which allows solvent extraction to occur on a diatomaceous earth inert support, has also become available in a 96-well plate format. The Extrahera system (Biotage) enables automation of SLE by carrying out all of the pipetting and extraction steps required. In the NWLP laboratory, this system is used for the extraction of patient samples for vitamin D measurement by LC-MS/MS. A sample throughput of up to 50,000 samples per annum is achieved with capacity remaining for additional extractions for use in other LC-MS/MS applications. The system is robust and reliable with good pipetting precision and uses disposable pipette tips, thus avoiding sample carry over. Figure 2 depicts the Tecan Freedom Evo 200 and Biotage Extrahera liquid handlers in use in the NWLP laboratory.
In some manufacturers’ LC-MS/MS systems, on-line sample preparation and extraction is enabled by use of turbo flow or 2D chromatography. On-line protein precipitation and SPE is also now available using the Clinical Laboratory Automated sample preparation Module (CLAM)-2000 (Shimadzu Corporation) [3] and the Rapidfire 365 MS system (Agilent) [4] respectively. These latter examples most closely resemble the immunoassay workflow, whereby samples are introduced into the analytical system without any sample preparation or pre-treatment.
Increased throughput
Increased throughput can be achieved through the use of column and sample managers, allowing multiple assay batches to be queued up for overnight analysis of different LC-MS/MS assays. LC multiplexing enables multiple columns to be coupled to one tandem mass spectrometry system, maximizing the MS detection capability. In this approach, the use of quaternary solvent pumps in the LC enables column switching between different columns using different mobile phases. Finally there is analyte multiplexing, which can use manufacturers’ kits or in-house laboratory developed tests (LDTs). This approach enables multiple analytes to be detected in a single chromatographic separation by the use of multiple reaction monitoring for MS/MS detection. Perkin Elmer and Chromsystems both provide kits enabling the simultaneous measurement of multiple steroid hormones within a single assay panel. In the NWLP laboratory an in-house LDT steroid panel for the simultaneous measurement of androstenedione, 17-hydroxyprogesterone and testosterone has been implemented. This multiplexed assay has replaced the previous stand-alone assays for these analytes, thus increasing throughput and offering faster turnaround time. The assay utilizes off-line SPE using Waters Oasis PRiME HLB 96-well plates and the Tecan Freedom Evo 200 automated liquid handler [5].
Improved integration
Improved integration can be achieved by the use of bi-directional interfacing between the LIMS and the LC-MS/MS instrument software. Nowadays, manufacturers of LC-MS/MS systems offer customer support to allow their systems to be interfaced to the LIMS. One example is the MassLynx LIMS interface (Waters), which enables both worklist download and results upload. The MassLynx LIMS interface is accessed via the LC-MS/MS system software allowing sample worklists, created by barcode scanning of the patient samples, to be imported directly. Following peak integration and analyte quantitation the results are directly transmitted from the LC-MS/MS to the LIMS via an HL7 interface. This avoids the need for manual transcription thus saving a great deal of staff time and eliminating transcription errors.
The ultimate aim of LC-MS/MS integration is to achieve complete integration of LC-MS/MS instruments into the automated workflow of high-throughput routine clinical laboratories. With the recent launch of the Cascadion LC-MS/MS analyser (Thermo Fisher Scientific) this ultimate aim has now been achieved [6]. This analyser offers a complete LC-MS/MS solution including primary blood tube sampling, on-board sample extraction, LIMS connectivity and a random access workflow enabling the provision of a 24/7 service. Traceable manufacturer’s kits are offered for the measurement of a panel of immunosuppressant drugs, testosterone and vitamin D with further assay kits in the development pipeline. The Cascadion analyser is shown in Figure 3.
Summary
LC/MS-MS automation and integration is now a reality, allowing faster sample processing and improved turnaround time, as well as offering increased staff productivity, improved quality and reduced error rate. Staff time is liberated for further service development, allowing the more rapid introduction of validated in-house LDTs into the assay repertoire. Finally there is the possibility of complete analyser integration allowing routine, high-throughput analysis, as is already the standard approach for the common immunoassay platforms. This exciting development will support the more widespread adoption of LC-MS/MS in the routine clinical laboratory by offering complete automation and integration, overcoming the barriers discussed in this article and enabling the inherent advantages of LC/MS-MS in clinical laboratory practice to be more fully realized.
References
1. Jones AM, Honour JW. Unusual results from immunoassays and the role of the clinical endocrinologist. Clin Endocrinol Oxf 2006; 64: 234–244.
2. Zhang YV, Rockwood A. Impact of automation on mass spectrometry. Clin Chim Acta 2015; 450: 298–303.
3. Shimadzu. CLAM-2000. Fully automated sample preparation module for LCMS. (https://www.shimadzu.com/an/lcms/clam/index.html).
4. Jannetto PJ, Langman LJ. High-throughput online solid-phase extraction tandem mass spectrometry: Is it right for your clinical laboratory? Clin Biochem 2016; 49: 1032–1034.
5. Williams EL. LC-MS/MS measurement of serum steroids in the clinical laboratory. Clinical Laboratory International 2017; Sept: 18–20.
6. ThermoFisher Scientific. Cascadion SM Clinical Analyzer (www.thermofisher.com/cascadion).
The author
Emma L. Williams PhD, FRCPath
North West London Pathology, Imperial College Healthcare NHS Trust, London, UK
E-mail: emma.walker15@nhs.net