Stem cell-derived ‘hematoids’ generate blood cells without yolk sac
Scientists at the University of Cambridge have created three-dimensional embryo-like structures that produce blood stem cells through a naturally occurring developmental process, eliminating the need for supplementary growth factors. The innovation, termed ‘hematoids’, mimics human embryonic blood development and could transform approaches to regenerative medicine and disease modelling.
Self-organising structures replicate early human development
The research team developed hematoids from human pluripotent stem cells, which self-organise into complex three-dimensional structures containing multiple tissue types. Unlike other existing methods for generating human blood stem cells in the laboratory, which require a cocktail of extra proteins to support the stem cells’ growth and development, the new method mimics the natural developmental process.
After approximately two weeks in culture, the structures spontaneously began producing blood cells, evidenced by visible red pigmentation. “It was an exciting moment when the blood red colour appeared in the dish – it was visible even to the naked eye,” said Dr Jitesh Neupane, first author of the study from the University of Cambridge’s Gurdon Institute.
The structures differ fundamentally from actual human embryos, lacking several essential embryonic tissues including the yolk sac and placenta necessary for further development. This distinction ensures ethical compliance whilst enabling detailed study of blood formation processes.
The research was published in Cell Reports on 13 October 2025.
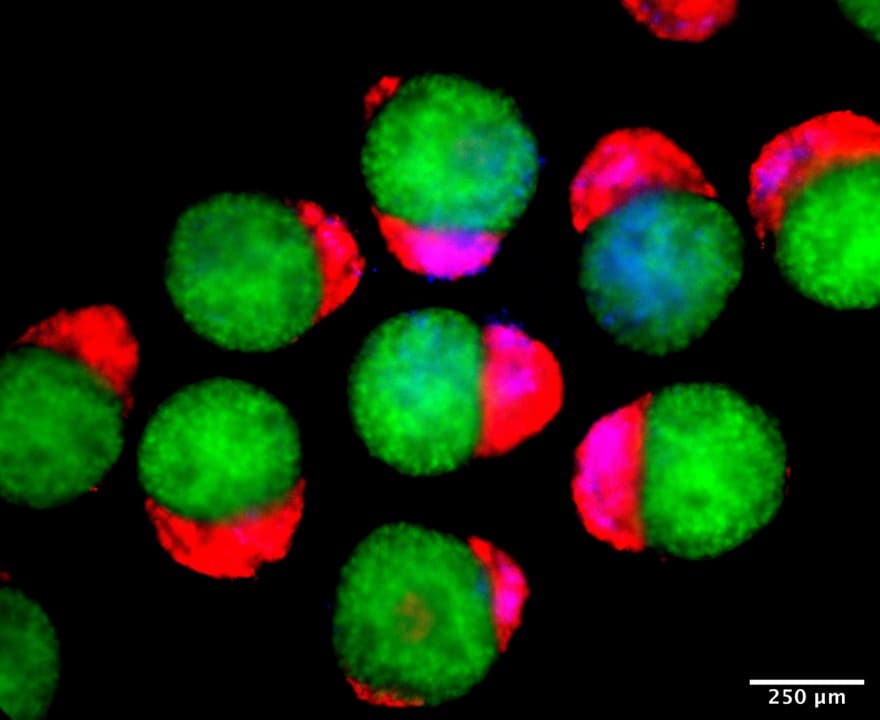
Day 4 hematoids displaying derivatives of the three germ layers – ectoderm (green), mesoderm (blue), and endoderm (red) – that establish the foundation of the human body. © Jitesh Neupane, University of Cambridge
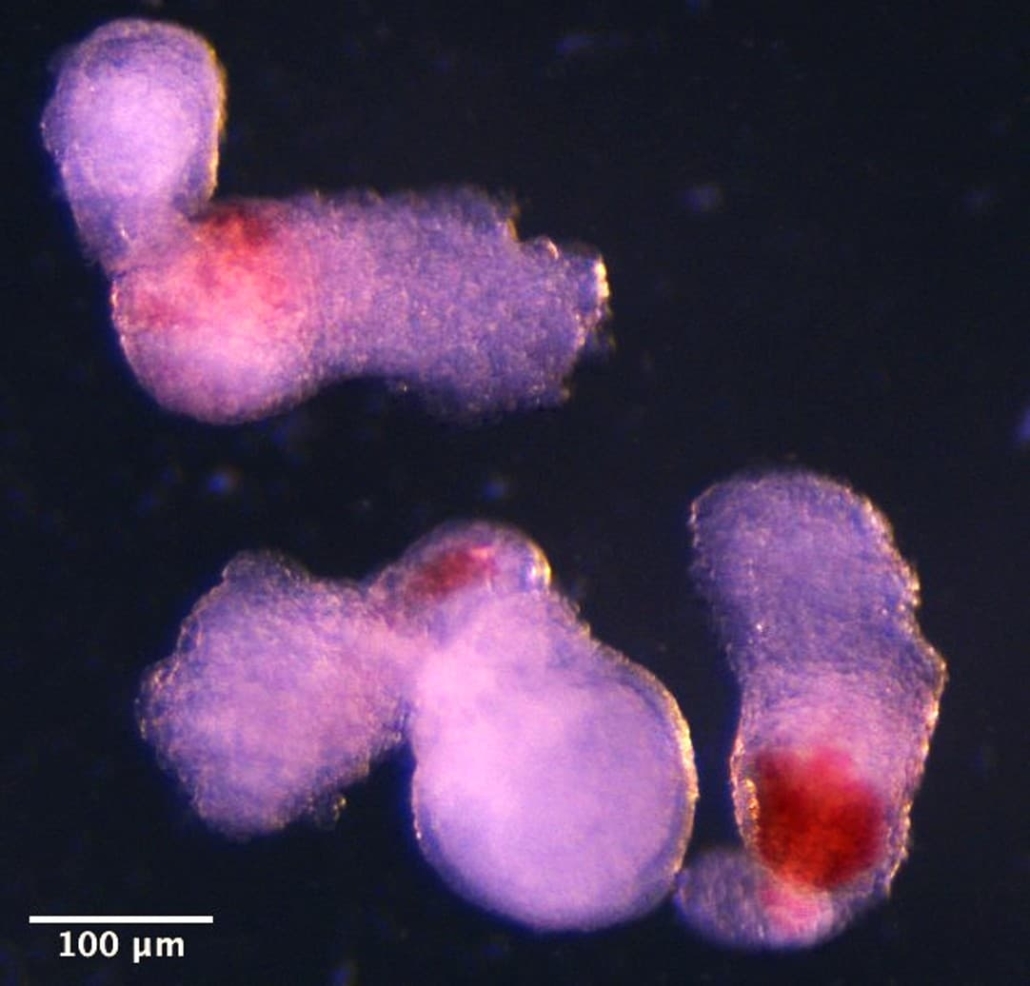
Day 14 hematoids developing visible red patches where blood is forming. © Jitesh Neupane, University of Cambridge
Hemogenic niches drive definitive blood development
The research team observed SOX17+RUNX1+ hemogenic buds within the structures, where endothelial-to-hematopoietic transition occurs. These hemogenic niches contain instructive factors including DLL4 and SCF, alongside restrictive factors such as FGF23, which regulate hematopoietic stem cell maturation.
The blood stem cells generated demonstrated capacity to differentiate into both myeloid and lymphoid lineages, confirming their identity as definitive rather than primitive haematopoietic cells. “Hematoids capture the second wave of blood development that can give rise to specialised immune cells or adaptive lymphoid cells, like T cells opening up exciting avenues for their use in modelling healthy and cancerous blood development,” explained Dr Geraldine Jowett, co-first author of the study.
Potential applications in personalised medicine
The hematoid system offers several advantages for research and potential clinical applications. Human stem cells used to create hematoids can be derived from any cell in the body, enabling production of patient-matched blood cells that would not trigger immune rejection.
Professor Azim Surani, senior author from the Gurdon Institute, stated: “This model offers a powerful new way to study blood development in the early human embryo. Although it is still in the early stages, the ability to produce human blood cells in the lab marks a significant step towards future regenerative therapies – which use a patient’s own cells to repair and regenerate damaged tissues.”
The structures develop to a stage roughly corresponding to weeks four to five of human embryonic development, a period normally inaccessible to direct observation following implantation. This temporal window proves crucial for understanding definitive haematopoiesis, which occurs in the aorta-gonad-mesonephros region around Carnegie stage 14.
Endogenous factors support blood cell maturation
The research demonstrates that definitive haematopoietic stem cell development in hematoids occurs through factors produced endogenously by the hemogenic niche, eliminating requirements for exogenous cytokine supplementation. The system successfully recapitulates critical features of human foetal haematopoiesis.
Experimental manipulation of signalling pathways revealed that transforming growth factor beta-1 inhibition proved essential for establishing hemogenic niches. Early inhibition prevented splanchnic mesoderm development and abolished hemogenic activity, whilst appropriately timed intervention enabled robust blood cell production.
The team identified specific molecular interactions governing blood development through computational analysis. Endothelial cells expressed key ligands including DLL4 and stem cell factor (SCF), which interacted with receptors on developing blood cells. Perturbation experiments using recombinant FGF23, DLL4 inhibition, and c-KIT blockade confirmed the functional importance of these signalling pathways.
Regulatory compliance and future directions
The research complied with clear regulations governing stem cell-based models of human embryos, with all work receiving approval from ethics committees before proceeding and undergoing peer review.
The scientists patented their work through Cambridge Enterprise, facilitating potential commercial development of the technology. Future applications may include drug screening, investigation of early blood and immune development, and modelling of haematological disorders including leukaemia.
The blood cells in hematoids expressed both embryonic and adult haemoglobin markers, suggesting representation of transitional erythropoietic states occurring during human development. This cellular heterogeneity reflects the natural switch from embryonic to foetal globins observed in developing embryos.
Reference
Neupane, J., Jowett, G. M., Wu, B., et. al. (2025). A post-implantation model of human embryo development includes a definitive hematopoietic niche. Cell Reports, 116373. https://doi.org/10.1016/j.celrep.2025.116373

