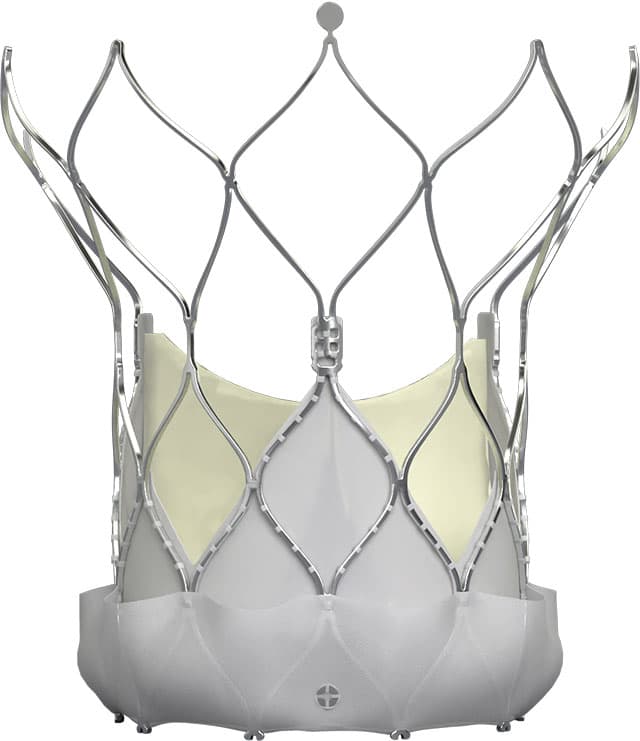
Abbott expands Navitor TAVI system indication in Europe
Abbott has received CE mark approval in Europe for an expanded indication of its Navitor™ transcatheter aortic valve implantation (TAVI) system, significantly broadening treatment options for patients with symptomatic, severe aortic stenosis. The minimally invasive device is now approved for patients at low or intermediate surgical risk, complementing its existing approval for high and extreme-risk patients obtained in 2021.
VANTAGE trial demonstrates robust clinical outcomes
The expanded indication is supported by compelling safety and effectiveness data from the VANTAGE study, presented as a late-breaking session at the European Society of Cardiology (ESC) Congress 2025 in Madrid and simultaneously published in JACC: Cardiovascular Interventions. The trial results demonstrate exceptional performance across multiple clinical endpoints.
“The VANTAGE study provides the scientific backbone for expanding Navitor’s indication to low- and intermediate-risk patients. The data are exceptional across both populations, confirming that the Navitor valve performs precisely as designed,” said Nicolas van Mieghem, M.D., medical director of the department of interventional cardiology at the Thoraxcenter, Erasmus University Medical Centre, Netherlands, and principal investigator of the VANTAGE trial.
Clinical endpoints exceed safety benchmarks
The VANTAGE trial’s 12-month follow-up data revealed impressive clinical outcomes. Among the first 262 patients, all-cause mortality or fatal stroke/stroke with disability occurred in only 2.3% of cases, demonstrating excellent safety profiles. The study achieved a 97% technical success rate with no procedural deaths recorded.
Effectiveness measures proved equally compelling, with no patients experiencing moderate or greater paravalvular leak (PVL) at 30 days. Only 13.6% of patients demonstrated mild PVL, a rate considered low according to established clinical benchmarks. The device maintained sustained haemodynamic performance throughout the 12-month follow-up period.
Strategic implications for structural heart interventions
The approval addresses a critical clinical need for patients with aortic stenosis, a condition characterised by narrowing of the aortic valve opening that restricts blood flow. Left untreated, this progressive condition can lead to heart failure and death. The Navitor system replaces the diseased valve through a minimally invasive procedure delivered via a small leg incision.
“Navitor is a strong example of how Abbott continues to evolve its structural heart portfolio to meet the growing demand for minimally invasive alternatives to open-heart surgery,” said Sandra Lesenfants, senior vice president of Abbott’s structural heart business. “Aortic stenosis is a life-threatening condition that can progress rapidly, and this expanded indication for Navitor means that patients have more options that can help reduce their symptoms and improve their lives.”
Enhanced guidelines support transcatheter interventions
Concurrent with the Navitor approval, ESC Congress 2025 witnessed updated guidelines for valvular heart disease management. The guidelines elevated mitral valve transcatheter edge-to-edge repair (TEER) from a treatment that should be considered (IIa) to a recommended treatment (Class Ia) for selected patients with severe functional mitral regurgitation. Similarly, tricuspid valve TEER received upgraded recommendations from IIb to IIa classification.
The Navitor TAVI system currently maintains approval in the United States for treating symptomatic, severe aortic stenosis patients at high or extreme surgical risk.
- For more information, visit: abbott.com