Bayer submits low-dose MRI contrast agent gadoquatrane for Japan approval
Bayer has submitted a groundbreaking marketing authorisation application to Japan’s Ministry of Health, Labour, and Welfare for gadoquatrane, an investigational contrast agent that could significantly improve MRI safety protocols. The macrocyclic agent delivers a remarkable 60% reduction in gadolinium exposure compared to standard-of-care agents, addressing critical concerns about cumulative gadolinium retention in patients requiring multiple imaging studies.
Currently, no low-dose MRI contrast agents are available in Japan, making this submission particularly significant for the country’s healthcare system, which performs approximately 20 million MRI procedures annually using the world’s highest per-capita concentration of scanners.
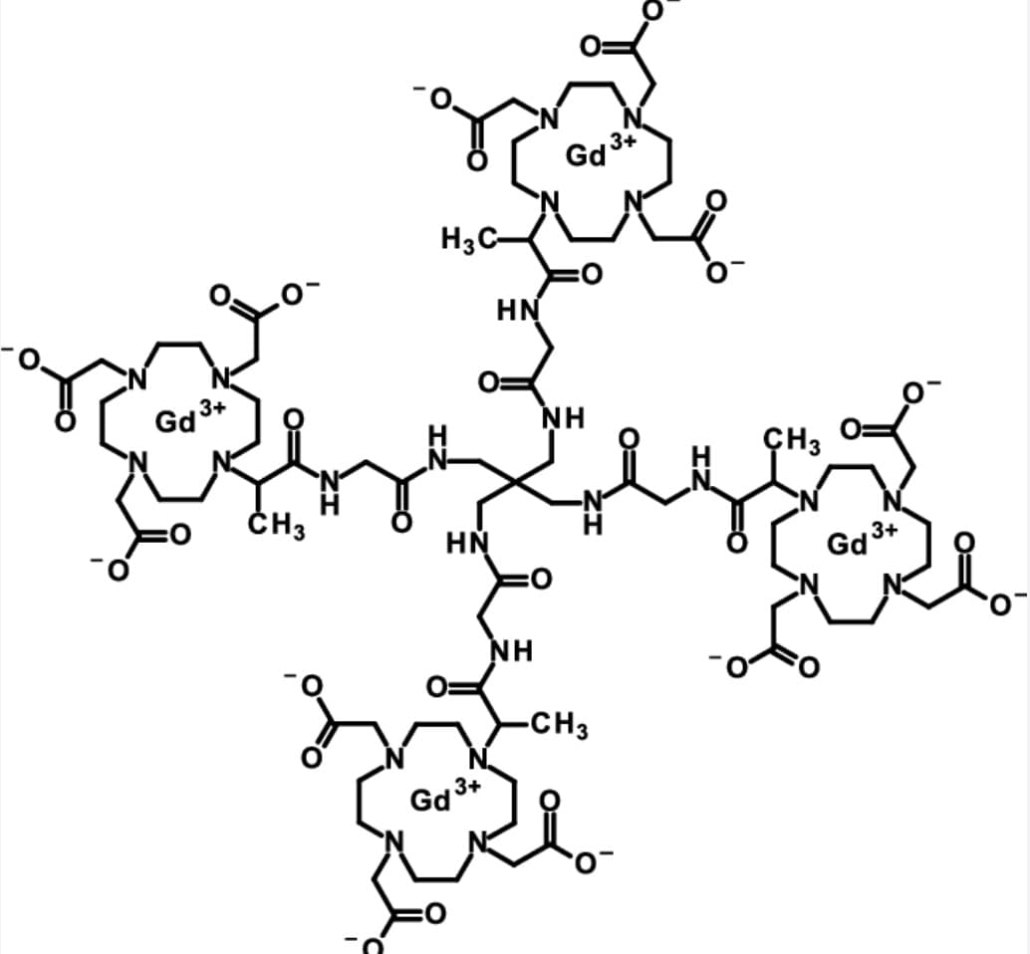
gadoquatrane
Transforming patient safety through dose reduction
The submitted dose of 0.04 mmol gadolinium per kilogram body weight represents a paradigm shift in contrast-enhanced imaging. This substantial reduction addresses growing clinical concerns about gadolinium accumulation, particularly in vulnerable populations requiring frequent surveillance imaging.
“As a leader in radiology, we are committed to bringing forward innovations for the benefit of patients, including potential options to reduce the gadolinium dose,” said Dr Konstanze Diefenbach, Head of Radiology Research & Development at Bayer. “If approved, gadoquatrane would offer radiologists in Japan a low-dose option to assist in diagnosing their patients. A low-dose option could be particularly valuable for patients undergoing multiple MRI examinations in their lifetime, as well as for children.”
The application encompasses comprehensive indications across all body regions and the central nervous system for adults and paediatric patients, including neonates – addressing Japan’s ageing population and rising chronic disease burden, particularly cancer and cardiovascular conditions.
Robust clinical validation demonstrates efficacy
The regulatory submission builds upon compelling evidence from the pivotal Phase III QUANTI programme, which evaluated gadoquatrane’s performance across diverse clinical scenarios. The comprehensive trials – QUANTI CNS (Central Nervous System), QUANTI OBR (Other Body Regions), and QUANTI Pediatric – enrolled 808 patients across 15 countries, with 27 Japanese sites contributing crucial data.
Positive topline results, announced in January 2025, demonstrated that gadoquatrane met both primary and secondary efficacy endpoints for visualisation parameters and lesion detection, achieving non-inferior performance compared to standard macrocyclic contrast agents.
Innovative molecular architecture enables breakthrough performance
Gadoquatrane’s distinctive tetrameric structure combines enhanced stability with high relaxivity, enabling effective contrast enhancement at significantly reduced gadolinium concentrations. This molecular innovation represents a major advancement in contrast agent chemistry, potentially transforming imaging protocols for patients with renal compromise or those requiring serial studies.
The safety profile remained consistent with established macrocyclic agents, with low incidence of intervention-related adverse events – a critical finding for clinical adoption.
This Japan submission marks the first global marketing authorisation application for gadoquatrane, with additional international regulatory submissions planned for coming months. The absence of low-dose alternatives in Japan’s market underscores the potential clinical impact of this breakthrough technology.
- For more information, visit: https://pharma.bayer.com

