Elixir Medical launches new intravascular lithotripsy system in Europe
Elixir Medical’s LithiX Hertz Contact IVL system receives CE Mark approval for treating calcified coronary lesions without external energy source.
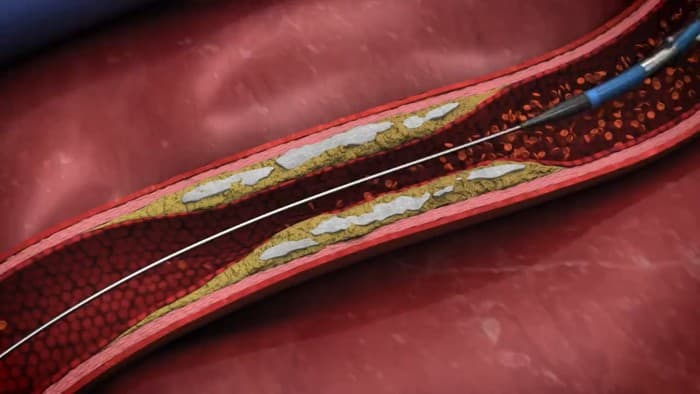
Elixir Medical has launched its LithiX Hertz Contact (HC) Intravascular Lithotripsy System (IVL) in Europe following EU MDR CE Mark approval. The novel calcium-modifying technology targets moderate to severely calcified coronary artery lesions without requiring an external energy source, expanding the company’s cardiovascular disease treatment portfolio.
The first procedures were successfully performed by specialists at leading European cardiac centres, including Dr Stefano Galli at Centro Cardiologico Monzino in Milan, Professor Eric Van Belle at Lille University Hospital, and Professor Nikos Werner at Hospital Barmherzigen Bruder Trier in Germany.
How the technology works
Unlike conventional IVL systems, the LithiX platform employs a mechanical approach based on the Hertz Contact Stress principle. The transcatheter device features multiple low-profile metal hemispheres distributed across a semi-compliant balloon surface. When inflated at low pressure, these hemispheres create localised contact points with calcium deposits, generating amplified force to create fractures in the calcified tissue.
“Lithotripsy, introduced in urology a few decades ago, has evolved to encompass mechanical and energy-based mechanisms of action to fragment calcium deposits while minimising injury to soft tissue,” explained Motasim Sirhan, CEO of Elixir Medical. “With LithiX, we created the first mechanical IVL platform capable of treating broad calcified lesion morphologies with great final outcomes, while minimising injury to adjacent non-calcified vessel.”
Clinical experience and outcomes
The technology appears to address a critical challenge in percutaneous coronary interventions (PCI), where calcium modification is essential for optimal stent deployment and procedural success.
“Optimal calcium modification is very important in achieving good PCI outcomes, and LithiX Hertz Contact IVL fulfils that goal as a simple-to-use and versatile option with excellent clinical performance,” said Dr Stefano Galli, Interventional Cardiologist at Centro Cardiologico Monzino in Milan.
Professor Nikos Werner from Hospital Barmherzigen Bruder Trier commented: “We are excited to adopt LithiX in our everyday practice as a very deliverable tool designed to safely modify calcified lesions that is easy to use with high degree of precision and control.”
The company recently presented six-month data from the PINNACLE I study, which evaluated the safety, effectiveness and performance of the LithiX HC-IVL System. The prospective, multicentre, single-arm clinical study included 60 patients across seven sites in Belgium and the Netherlands, with an imaging subset of 32 patients who underwent optical coherence tomography (OCT) assessment.
Imaging results demonstrate effectiveness
The intravascular imaging sub-study demonstrated the system’s effectiveness across various calcified lesion types, including concentric and eccentric calcium deposits and lesions with calcified nodules. Stent expansion at the Minimum Stent Area location was 101.38% in eccentric lesions and 93.95% in concentric lesions. In lesions with calcified nodules, stent expansion at the maximum calcium site was 102.81%.
“In our first cases, we have seen confirmation of the recent PINNACLE study outcomes with LithiX HC-IVL in treating calcified coronary lesions with a great procedural result in eccentric and concentric calcium,” noted Professor Eric Van Belle, Head of the Interventional Cardiology Department at Lille University Hospital.
Regulatory status
The LithiX HC-IVL system has received CE Mark approval under the EU Medical Device Regulation. It is not currently available for sale in the United States.
Elixir Medical Corporation is a privately held company based in Milpitas, California, developing technologies for coronary and peripheral artery disease treatment. The company was recently named to Fast Company’s list of the World’s Most Innovative Companies of 2025.
- For more information, visit: elixirmedical.com

